Abstract
Increased expression of forkhead box O (Foxo) transcription factors were reported in cultured myotubes and mouse limb muscle with corticosteroid (CS) treatment. We previously reported that administration of CS to rats resulted in muscle fiber atrophy only by day 7. The aim of this study, therefore, was to evaluate the time-course changes in the expression of Foxo transcription factors and muscle-specific ubiquitin E3 ligases in rat limb muscle following CS administration. Triamcinolone (TRI; 1 mg · kg−1 · day−1 im) was administered for 1, 3, or 7 days. Control (CTL) rats were given saline. Muscle mRNA was analyzed by real-time RT-PCR. Compared with CTL, body weights of TRI-treated animals decreased by 3, 12, and 21% at days 1, 3, and 7, respectively. Muscle IGF-1 mRNA levels decreased by 33, 65, and 58% at days 1, 3, and 7 in TRI-treated rats compared with CTL. Levels of phosphorylated Akt were 28, 50, and 36% lower in TRI animals at these time points. Foxo1 mRNA increased progressively by 1.2-, 1.4-, and 2.5-fold at days 1, 3, and 7 in TRI animals. Similar changes were noted in the expression of Foxo3a mRNA (1.3-, 1.4-, and 2.6-fold increments). By contrast, Foxo4 mRNA was not significantly changed in TRI animals. With TRI, muscle atrophy F box/Atrogin-1 increased by 1.8-, 4.1-, and 7.5-fold at days 1, 3, and 7 compared with CTL rats. By contrast, muscle RING finger 1 increased only from day 7 (2.7-fold). Gradual reduction in IGF-I expression with TRI over the time series paralleled that of Akt. These findings are consistent with a progressive stimulus to muscle protein degradation and the need to process/remove disassembled muscle proteins via the ubiquitin-proteasome system. Elucidating the dynamic catabolic responses to CS challenge is important in understanding the mechanisms underlying muscle atrophy and therapeutic measures to offset this.
Keywords: atrogenes, muscle fiber atrophy, insulin-like growth factor-I
several medical conditions, including pulmonary, rheumatologic, and transplantation states, commonly require the use of corticosteroids (CS) for optimal medical management in the setting of acute exacerbations, induction of disease remission, immunosuppression, and/or maintenance therapy (2, 7, 37, 43, 59). Despite the obvious medical benefits of systemic CS therapy, there are significant adverse side effects associated with its use (39). In particular, the use of CS has been reported to adversely impact on skeletal muscle structure and function in patients with both acute and chronic disorders (3, 5, 11–13, 60). For example, CS was reported to cause respiratory and limb muscle weakness, reduced muscle mass, and muscle fiber atrophy in patients with chronic obstructive pulmonary disease (COPD), with associated worse survival (3, 11–13). In an animal model, we have previously reported on the impact of CS on gastrocnemius muscle fiber cross-sectional areas in both the deep (red) and superficial (white) portions, with significant atrophy of major fiber types, particularly type II fibers (35).
CS leads to muscle atrophy by adversely impacting on both the synthetic and protein degradation arms of protein turnover (1, 24, 28, 42, 57, 63, 65). The reduction in protein synthesis with CS administration is related to a diminished rate of protein translation initiation and not limited by impairment of translational elongation or transcription (56, 57). Enhanced protein breakdown with CS is in large part due to ATP-dependent ubiquitin-mediated proteolysis (1, 65). The phosphatidylinositol 3-kinase (PI3K)/Akt (serine/threonine kinase also known as protein kinase B) pathway is important in maintaining the balance between protein synthesis and degradation, with Akt functioning as a key “anchor” (25, 50, 51, 58). Decreased activation of Akt in cultured myotubes was shown to activate forkhead box O (Foxo) transcription factors, with subsequent enhanced expression of muscle-specific ubiquitin E3 ligases, such as muscle atrophy F box (MAFbx; also known as Atrogin-1) and muscle RING finger-1 (MuRF1) (51, 58). By contrast, phosphorylation of Akt by insulin or insulin-like growth factor-I (IGF-I) has been shown to phosphorylate and inhibit Foxo transcription factors by shuttling them away from the nucleus to the cytosol, thus blocking induction of MAFbx or MuRF1, with reduced muscle proteolysis (38, 50, 51, 58, 61).
To date, most of the data reported for CS influences on signal transduction pathways have been obtained in cell culture, with a paucity of data in animal models of steroid-induced muscle atrophy. Of those reported, all have employed supratherapeutic doses of CS with regimens of 5 to 80 times that of clinically high dosing schedules (e.g., 1, 8, 18, 19, 30, 35, 38, 52, 66). Further, there are limited time-series data in vivo evaluating the interactive expression of Akt, Foxo transcription factors, and muscle-specific ubiquitin E3 ligase interactions. For example, Auclair et al. (1) evaluated CS effects on the rat limb muscles over 3 days. In the extensor digitorum longus (EDL), they reported an early peak in the polyubiquitin transcripts UbB and UbC at day 1 with a second larger peak at day 3. E2-14k (an ubiquitin conjugating enzyme) peaked at day 2. Sassoon and coworkers (52) used supraphysiological doses of CS in rabbits and reported decrements in diaphragm muscle IGF-I of similar degree over 1, 2, and 3 days with increments in MAFbx over the same time course. While several markers of limb muscle protein turnover in vivo (e.g., IGF-I, Akt, MAFbx, MuRF1, Foxo1, and Foxo3a) have been reported following the administration of CS, the data generally represent only one point in time varying from 6 h to 13 days (e.g., 19, 30, 38, 62, 66). Of interest, a gene array study using rat gastrocnemius muscle at day 3 after high-dose CS revealed cathepsin L as a significant early mediator of proteolysis and not markers of the ubiquitin proteasome system (30). The varying methodological approaches highlighted above preclude firm conclusions being drawn regarding the complex and dynamic biochemical events following CS administration.
The aim of this study, therefore, was to employ a clinically relevant dose of CS for a time course analysis examining the expression of muscle-specific ubiquitin E3 ligases and Foxo transcription factors in an animal model of limb muscle atrophy due to CS. This would enable us to determine the expression of individual signals and their potential correlations with downstream effectors. We hypothesize that changes in Foxo transcription factors and the ubiquitin E3 ligases on which they act are linked, but that the upregulation of their individual components may differ both temporarily and in extent. These studies may further elucidate dynamic complex mechanisms underlying the aggregate effects of CS-induced muscle atrophy in the animal model.
METHODS
Animal Groups
Young adult male Sprague-Dawley rats (initial body wt = 280 g) were divided into two groups: 1) a control group (CTL; n = 21), and 2) a group receiving triamcinolone (TRI; n = 21). TRI was administered by intramuscular injection (1 mg · kg−1 · day−1) over 1, 3, or 7 days. CTL animals received daily saline injection over the same duration. All injections were provided at the same time each day (between 10:00 and 11:00 AM). Food and water were provided to both groups ad libitum. Animals were housed with a 12:12-h dark:light cycle and ambient temperature maintained at 22°C. The research protocol was approved by the Burns and Allen Research Institute Animal Care and Use Committee of Cedars-Sinai Medical Center.
Muscle Biochemical Studies
mRNA extraction.
Total RNA was extracted from gastrocnemius samples with TRIzol reagent (Invitrogen, Carlsbad, CA) according to manufacturer's protocol. Quality and concentrations of total RNA were determined with a spectrophotometer (SmartSpec 3000, Bio-Rad, Hercules, CA). Samples were stored at −80°C in RNase-free water until analysis. Two micrograms of total RNA was reverse transcribed (RT) using oligo(dT) primers (Invitrogen) and Omniscript RT kit (Qiagen, Valencia, CA), and reactions yielded 20 μl of first-strand cDNA.
Oligonucleotides.
The primers for IGF-I, transcription factors Foxo1 (also known as FKHR), Foxo3a (also known as FKHRL1), and Foxo4 (also known as AFX), muscle-specific ubiquitin E3 ligases MAFbx/Atrogin 1 and MuRF1, as well as the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed based on published rat cDNA sequences using Primer3 on the World Wide Web for general users and for biologist programmers (2004, Whitehead Institute for Biomedical Research, Cambridge, MA; see Ref. 49). Sequences of primers used for cDNA synthesis and real-time RT-PCR analysis, along with their accession number and product length, are described in Table 1.
Table 1.
Primers used for cDNA synthesis and real-time RT-PCR analysis
| Primer Name | Accession No. | Primer Sequence (5′→3′) | Product Length, bp |
|---|---|---|---|
| GAPDH | DQ403053 | Sense: ATG ACT CTA CCC ACG GCA AGT T | 51 |
| Antisense: TCC CAT TCT CAG CCT TGA CTG T | |||
| MAFbx | AY059628 | Sense: CTA CGA TGT TGC AGC CAA GA | 138 |
| Antisense: AAA GTGT CAG TAT CCA TGG CG | |||
| MuRF1 | AY05627 | Sense: GGG GAG GAA GAC AAA GAG GA | 137 |
| Antisense: TCT CCA TAG CAT TTC CGT CC | |||
| Foxo1 | NM_012560 | Sense: GAG GTG CAA TGT GGG AGA AT | 101 |
| Antisense: TTG AAT GAA ATG GCA AAG CA | |||
| Foxo3a | NM_201559 | Sense: TCT CCC GTC AGC CAG TCT AT | 112 |
| Antisense AGT CAC TGG GGA ACT TGT CG | |||
| Foxo4 | XM_233465 | Sense: GAC TGC GAG TCC ATC ATC CT | 70 |
| Antisense GGG CTG AGT CGA AGT TGA AG | |||
| IGF-1 | X06107 | Sense: TTG CTT CCG GAG CTG TGA TC | 51 |
| Antisense: CAG CGG ACA CAG TAC ATC TCC A |
See text for definition of abbreviation of primer names.
Real-time RT-PCR.
Primer efficiency tests were performed to compare amplification efficiency between the target genes and the endogenous reference gene (GAPDH). A PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) with V1.76A software was used to set the reaction plate design to perform the real-time PCR. The following experimental conditions were set: 50°C for 2 min, 95°C for 10 min, 95°C for 30 s, 60°C for 1 min, and set for 40 cycles. For each well, 65 ng of cDNA template was used with the master mix (Applied Biosystems) containing SYBR green in a volume of 25 μl. To compare the relative mRNA expression between control and experimental groups, the comparative threshold cycle (CT; the fractional cycle number at which the amount of amplified target reaches a fixed threshold) method was used. This relative quantification is achieved using the following arithmetic formula:
where ΔCT is the difference in threshold cycles for target and reference. The fold change in mRNA expression as a result of experimental condition was calculated using the following formula:
The amount of target, normalized to the endogenous reference and relative to the calibrator, is given by the following formula:
(Further details pertaining to the derivation of the formula describing the exponential amplification of PCR are available in User Bulletin 2: ABI PRISM 7700 Sequence Detection System.)
Thus the final result is the fold change in mRNA expression relative to that of the control group.
Protein extractions.
Soluble protein was extracted from 50-mg samples of the gastrocnemius muscle in a 1:10 ratio of cold cell lysis buffer (Cell Signaling Technologies, Beverly, MA) according to manufacturer's protocol. Homogenization was performed with a Polytron homogenizer, and homogenates were centrifuged at 14,000 rpm. The supernatant was aliquoted in microcentrifuge tubes. Protein concentration was determined using a commercial protein assay kit (Bio-Rad) based on the Bradford (6) method and measured with a spectrophotometer (SmartSpec 3000, Bio-Rad).
SDS-PAGE and Western blotting.
Samples were boiled and cooled before being used for electrophoresis. Protein extracts were loaded on 4–20% linear gradient gels. Proteins were then electrophoretically transferred to nitrocellulose membranes. Rabbit polyclonal antibodies directed at total Akt and phosphorylated Akt at the COOH terminus (Ser473) were from Cell Signaling Technology. Blots were incubated with primary antibodies at 4°C overnight, washed, and incubated with an appropriate secondary antibody at room temperature for 1 h. The blots were visualized following development with enhanced chemiluminescence reagents (ECL streptavidin-horseradish peroxidase, Amersham, Piscataway, NJ) according to manufacturer's protocol. In a few instances blots were reused by exposing them to stripping buffer (Restore, Pierce Biotechnology, Rockford, IL) and reprobed with a different antibody. Blots were exposed to X-ray film in a cassette, the films scanned, and identified bands analyzed by densitometry using a Kodak Analysis System. Western blot data from the CS group were expressed relative to measured mean values from the CTL group.
Statistical Analysis
The distribution of all data was tested for normality, and statistical analysis was then performed using a one-way ANOVA (SigmaStat v. 2.0, Jandel, Richmond, CA) to compare differences between the independent groups. If a significant interaction was found, post hoc analysis (Newman-Keuls test) was used to compare differences in independent groups. An α-level of 0.05 was used to determine significance. Values are means ± SE.
RESULTS
Body Weights and Muscle Mass
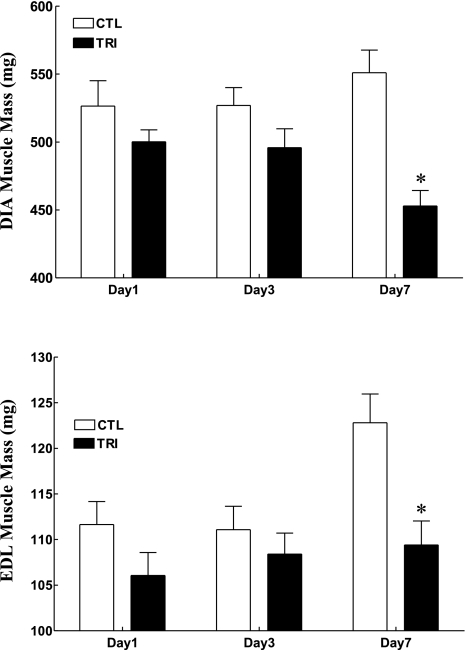
CS administration induced a gradual and significant decrement in body weight over the 7-day period, with mean body weights for CS animals down 3, 12, and 17% at days 1 (P = 0.09), 3 (P < 0.0001), and 7 (P < 0.0001), respectively, compared with CS animals at day 0 (Fig. 1). Of interest, the slope of body weight decrement over time was similar for CS animals at days 1 and 3, while for day 7 animals, the rate of weight loss was attenuated from day 5 (Fig. 1, top). In CTL animals, body weight increased slightly over the same time periods with a final weight gain of 5% (P = 0.12) at day 7 (Fig. 1). Thus the mean final body weight of CS-treated animals was 3, 12, and 21% lower at days 1 (P = 0.15), 3 (P < 0.01), and 7 (P < 0.001), respectively, compared with CTL animals (Fig. 1, bottom). Examples of gradual skeletal muscle atrophy with CS treatment are shown in Fig. 2 where the mean muscle masses of the diaphragm (Fig. 2, top) and extensor digitorum longus (Fig. 2, bottom) at day 7 were significantly decreased (P < 0.05) by 18 and 11%, respectively, compared with those of CTL animals.
Fig. 1.
Daily body weight change (top) over the experimental period and final body weights (bottom) in days 1, 3, and 7 control (CTL) and triamcinolone-treated (TRI) animal groups (n = 7 for each subgroup). Values are means ± SE. *Significantly different from CTL groups.
Fig. 2.
Final diaphragm (DIA; top) and extensor digitorum longus (EDL; bottom) muscle masses in days 1, 3, and 7 CTL and triamcinolone-treated animal groups (n = 7 for each subgroup). Values are means ± SE. *Significantly different from CTL.
Muscle IGF-I mRNA
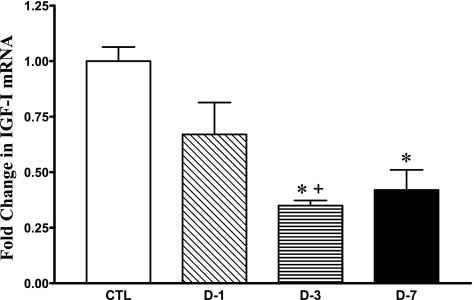
The muscle IGF-I mRNA abundance decreased by 33, 65, and 58% at days 1 (P = 0.1), 3 (P < 0.001), and 7 (P < 0.05), respectively, in CS-treated rats compared with CTL (Fig. 3). The abundance of IGF-1 mRNA at day 3 was also significantly lower than at day 1 with CS administration (P < 0.05; Fig. 3).
Fig. 3.
Change in skeletal muscle IGF-I mRNA abundance by real-time RT-PCR analysis in CTL (n = 8, drawn from all 3 time points) and days 1 (D-1; n = 7), 3 (D-3; n = 7), and 7 (D-7; n = 7) triamcinolone-treated animal groups. Values are means ± SE relative to mean CTL values. *Significantly different from CTL. +Significantly different from D-1.
Muscle Akt Protein Levels
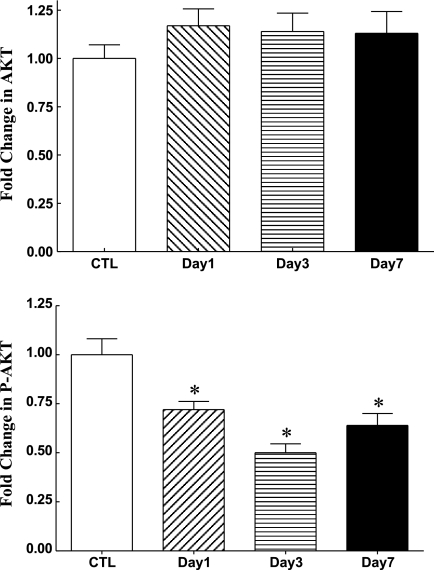
Analysis of muscle total Akt showed no change in its protein levels over time with CS treatment (Fig. 4). However, CS-treated rats exhibited a sustained decrease in the phosphorylated (P) form of Akt of 28% at day 1 (P < 0.01), 50% at day 3 (P < 0.0001), and 36% at day 7 (P < 0.005) compared with CTL (Fig. 4).
Fig. 4.
Western blot analysis of skeletal muscle total Akt (top) and phosphorylated (P) (Ser473) Akt (bottom) in CTL (n = 8) and days 1 (n = 7), 3 (n = 7), and 7 (n = 7) triamcinolone-treated animal groups. Values are means ± SE relative to mean CTL values. *Significantly different from CTL.
Muscle Foxo mRNA
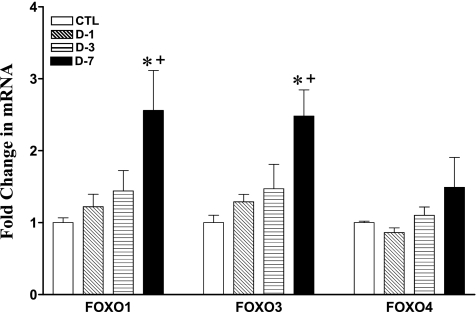
Muscle Foxo1 and -3a mRNA levels showed progressive change over time, while Foxo4 remain unchanged (Fig. 5). The abundance of Foxo1 mRNA increased progressively by 1.2-, 1.4-, and 2.5-fold at days 1, 3, and 7 (P < 0.5), respectively, in CS-treated animals (Fig. 5). Further, mRNA abundance at day 7 was significantly greater than at day 1 (P < 0.05; Fig. 5). Similar changes were noted in the expression of Foxo3a mRNA with 1.3-, 1.4-, and 2.6-fold (P < 0.05) increments. In addition, mRNA abundance at day 7 was significantly greater than at day 1 (P < 0.01; Fig. 5). By contrast, Foxo4 mRNA abundance was not significantly changed at all time points, although there was a trend for an increment at day 7 (1.5-fold increment; P = 0.2) in CS animals.
Fig. 5.
Change in skeletal muscle Foxo1, Foxo3a, and Foxo4 mRNA abundance by real-time RT-PCR analysis in CTL (n = 8) and days 1 (D-1; n = 7), 3 (D-3; n = 7), and 7 (D-7; n = 7) triamcinolone-treated animal groups. Values are means ± SE relative to mean CTL values. *Significantly different from CTL. +Significantly different from D-1.
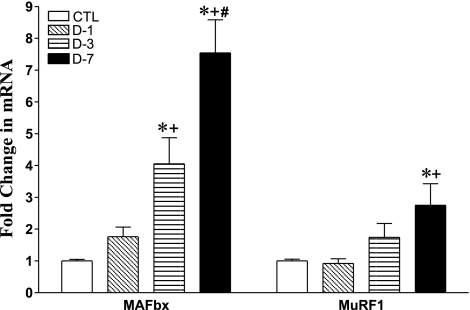
Muscle-Specific Ubiquitin E3 Ligase mRNA
The mRNA abundance of muscle-specific ubiquitin E3 ligases (MAFbx/Atrogin-1 and MuRF1) exhibited different time course increments at days 1, 3, and 7 (Fig. 6). With CS administration, MAFbx (Atrogin-1) increased by 1.8-, 4.1-, and 7.5-fold at days 1 (P = 0.1), 3 (P < 0.01), and 7 (P < 0.001), respectively, compared with CTL rats (Fig. 6). Furthermore, abundances were significantly greater at days 3 (P < 0.05) and 7 (P < 0.001) than at day 1 of CS-treated animals (Fig. 6). The mRNA abundance at day 7 was also significantly greater than at day 3 (P < 0.05) with CS administration.
Fig. 6.
Change in skeletal muscle-specific ubiquitin E3 ligases MAFbx and MuRF1 mRNA abundance by real-time RT-PCR analysis in CTL (n = 8) and days 1 (D-1; n = 7), 3 (D-3; n = 7), and 7 (D-7; n = 7) triamcinolone-treated animal groups. Values are means ± SE. *Significantly different from CTL relative to mean CTL values. +Significantly different from D-1. #Significantly different from D-3.
By contrast, the increments in muscle RING finger 1 (MuRF1) were significantly less than observed for MAFbx. Further, there was only a trend for MuRF1 abundance to increase by day 3 (1.8-fold; P = 0.07), with a significant rise noted only at day 7 (2.7-fold; P < 0.05) (Fig. 6). Only at day 7 was MuRF1 abundance greater than at day 1 (P < 0.05).
DISCUSSION
This study highlights the time-course changes in several key signaling pathways in the gastrocnemius muscle of adult rats with the administration of a clinically relevant dose of CS. In this study, we elected a priori not to include a pair-fed (or pair weight) group. The major rationale was that we were interested in evaluating the aggregate influences of CS on limb muscle Foxo transcription factor expression using a clinical dosing regimen. The intent was not to analyze the separate actions of CS (direct and indirect) in mediating biochemical changes in muscle, but to evaluate the total/composite/aggregate response to CS administration, as occurs in the clinical scenario. A literature review limited to rats in which CS were given added to our belief that the impact of pair feeding (CS-induced decrement in food intake) in the short term has a minimal to no significant impact on muscle mass (albeit a greater impact on body weight). In the majority of papers, the dose of CS was significantly greater than that used in the present study and the duration of treatment varied greatly (from 5 to 10 days) with a single end point only (9, 15, 18, 41, 53). Nevertheless, these data support our rationale not to include a pair-fed group. For example, Moore et al. (41) reported no change in gastrocnemius, EDL, and diaphragm muscle masses in pair-fed rats compared with free-eating controls despite a 35, 28, and 28% decrease in muscle masses, respectively, in CS-treated rats. Food intake was reduced by 14.9% in the pair-fed group. Similarly, Dumas et al. (15) reported no significant change in gastrocnemius mass in pair-fed rats compared with free-eating controls, while steroid-treated animals had a 23% decrement in muscle mass. Of note, in the Moore study (41), body weight in pair-fed animals was attenuated 9% compared with controls, while it was reduced by 13% in the study by Dumas et al. (15). Other points of note were a clear dose response evident for muscle mass (and body weight) loss where this was tested (38), and our dosing regimen was much less than in the majority of rat studies. Further, we have shown that providing only 60% of normal food intake has no significant impact on body weight in the first 1, 3, and 7 days of a 21-day study in rats (34).
The main findings of this study were as follows. 1) There was a progressive decline in body weights starting as early as day 1 and following the same substantial decline thereafter. 2) Muscle IGF-1 mRNA abundance was significantly reduced by day 3 and remained markedly reduced at day 7. 3) The expression of phosphorylated (P)-Akt was significantly reduced as early as day 1 with further decrement by day 3. Levels remained significantly reduced at day 7. 4) Muscle mRNA abundance of Foxo1 and -3a were significantly elevated only by day 7, while no changes in Foxo4 were observed. 5) The time course changes in muscle-specific ubiquitin E3 ligases differed. Muscle mRNA abundance of MAFbx increased progressively but was significant only from day 3, while abundances of MuRF1 were elevated only at day 7. Of interest, the peak fold change for MAFbx greatly exceeded that of MuRF1.
CS and Muscle Atrophy
CS impact adversely on both arms of muscle protein turnover by reducing protein synthesis and enhancing protein breakdown. For example, the fractional synthesis rate of myosin heavy chains (the major skeletal muscle contractile protein) was reduced by 35% in the plantaris muscle of rats following CS administration (10). Further, a 60% increase in the excretion of urinary 3-methylhistidine (an index of myofibrillar breakdown) was reported in rats given CS (55). The major mechanism underlying reduced muscle protein synthesis is related to a diminished rate of translation initiation and thus efficiency (56, 57) with reduced translational capacity with more extended periods of CS administration (48, 57). By contrast, enhanced muscle protein breakdown with the use of CS is mediated in large part via the ATP-dependent ubiquitin-mediated proteolytic pathways (1). This disordered protein turnover culminates, as we previously reported, in significant muscle fiber atrophy in both limb and respiratory muscles (35). CS treatment significantly reduced the mean cross-sectional areas of both types I and II fibers in the deep (red) portion of the gastrocnemius as well as of type II fibers in the superficial (white) portion of the gastrocnemius and the diaphragm muscles (35).
Muscle IGF-1 and P-Akt
We have previously reported reduced levels of muscle IGF-1 in the diaphragm of rats following malnutrition of varying severity, another model of metabolic stress (including endogenous CS elaboration) and disordered protein turnover (34). Large doses of CS (methylprednisolone and triamcinolone) given to rats over 5 and 13 days, or rabbits over 3 days, were also reported to significantly reduce IGF-1 mRNA abundances in the gastrocnemius and/or diaphragm muscles (18, 52, 66). IGF-I mRNA abundance was also reduced in the gastrocnemius of mice given high-dose dexamethasone (19). In the present study, reduced abundance of muscle IGF-1 mRNA were seen by day 3, but using an 80-fold reduction in CS dose to that used in the study by Gayan-Ramirez and colleagues (18). This indicates that similar influences were still evident at doses of the CS that mirror clinical dosing regimens.
Reduced muscle IGF-1 likely plays an important role in CS-induced skeletal muscle wasting, as it impacts on important signaling pathways downstream of its receptor. This includes activation of the phosphatidylinositol-3-kinase (PI3K)/Akt pathway to suppress muscle proteolysis via the ubiquitin-proteasome and lysosomal pathways. (32, 50). In addition, the PI3K/Akt/GSK3β/β-catenin pathways have also been implicated in CS-induced protein breakdown and atrophy in myotubes (36) and in vivo, in rat limb muscles (54), with IGF-1 reversing the negative influences of CS on these signal transduction pathways. Further, as proof of concept, either systemic administration of IGF-1 (e.g., 17, 27) or local limb muscle IGF-1 gene transfer by electroporation techniques (53) prevents or attenuates CS-induced muscle atrophy.
P-Akt levels in the gastrocnemius in the present study were significantly reduced as early as 1 day following the administration of CS and remained down through day 7. The nadir was at day 3, where levels were <50% of controls. Similar reduction in P-Akt was recently reported in the tibialis anterior and gastrocnemius muscles of rats given dexamethasone for either 7 or 13 days (54, 66). Of interest, reduction in Akt activity has been shown to correlate with reduced levels (14), while local muscle overexpression of a constitutively active form of Akt prevented CS-induced muscle fiber atrophy (54). Thus the early and sustained reduction in P-Akt muscle expression in the present study would be expected to adversely influence both the synthetic and proteolytic (see below) arms of muscle protein turnover, resulting in early and progressive loss of muscle mass, as suggested by the effects on body mass over 7 days with CS administration.
Foxo Transcription Factors
The role members of the forkhead box O (Foxo) transcription factors play in several different models of muscle atrophy as well as their influences on muscle specific ubiquitin E3 ligases has recently been highlighted (e.g., 51, 58). Further, Foxo1, -3a, and -4 are expressed in skeletal muscle, and their role in myogenic processes, regulation of muscle metabolism, and proteolysis are the focus of much interest (22). In muscle cell cultures, activation of either Foxo1 or -3a has been linked to the expression of muscle-specific ubiquitin E3 ligases, in part via activation of the MAFbx/atrogin 1 promoter (Foxo3a; 51) or MuRF promoter (Foxo1; 62). Phosphorylation of the Foxo transcription factors inhibits their action by shuttling them away from the nucleus to the cytosol (61). For example, in C2C12 muscle cell culture, Zhao et al. (68) reported a marked decrement in phosphorylated Foxo1 following incubation with dexamethasone.
In the present study, assays were performed in an in vivo model of CS administration. This is important as much of the literature relates to excellent studies in muscle cell cultures, which have several drawbacks. These include the small fraction of myofibrillar protein content in cultured myocytes compared with their 60–70% (of total muscle protein) content in adult muscle (67). In addition, the bloodless preparation precludes the normal complex interactive physiological, metabolic, and genetic responses to the intervention, which could skew the results. For example, mRNA abundance for Foxo factors 1 and 3a were increased in the gastrocnemius muscle of rats treated with AICAR [an AMP-activated protein kinase (AMPK) agonist] but decreased in C2C12 muscle cell lines with the same intervention (44).
The present study demonstrated that the mRNA abundance of Foxo1 and -3a were significantly elevated only by day 7. This contrasts with significantly increased abundance of Foxo3a mRNA in the gastrocnemius of mice given dexamethasone (19). In the present study, significant and progressive body weight loss was evident as early as day 3. This suggests that the impact of the CS on the PI3K/Akt pathways early on, as shown by the reduced levels of P-Akt at day 1, likely influenced preformed Foxo transcription factors, with reduced phosphorylation and activation of the latter and shuttling of the transcription factors back to the nucleus and/or reduced cytosolic extrusion. The sustained influences of reduced P-Akt with CS administration may well have been the stimulus for increased transcription factor availability together with stimuli from other possible upstream metabolic regulators (e.g., AMPK) (29). It is also possible that the disordered state of protein turnover induced by CS impaired the normal synthesis of these factors and/or enhanced their ubiquitination and breakdown, thus promoting new factor generation.
In the present study, no changes were observed for Foxo4 at any time point, suggesting that this transcription factor is unlikely an important biochemical mediator of disordered protein turnover and loss of skeletal muscle mass in our model of CS administration. The functions of the various Foxo transcription factors are complex and diverse and still have to be fully defined. Of interest, a recently reported sepsis model, induced by cecal ligation and puncture (known to be accompanied by endogenous CS elaboration, acute muscle proteolysis, and muscle atrophy), also failed to show any changes in Foxo4 mRNA in the gastrocnemius muscle of the mice, despite significant increments in Foxo1 and -3 mRNA abundances (44). The actions of Foxo4 in skeletal muscle are unclear. Whether the transcription factor has a role in muscle oxidative stress protection and muscle metabolism still needs to be clarified (26). Compared with Foxo1 and -3a, Foxo4 is activated in skeletal muscle through JNK pathways rather than via PI3K/Akt signaling (as reported for all other tissues) (46).
Muscle-Specific Ubiquitin E3 Ligases
Degradation of muscle contractile proteins is predominantly mediated via the ATP-dependent ubiquitin-proteasome system (40). Recently, important muscle-specific ubiquitin E3 ligases have been described (MAFbx/atrogin-1 and MuRF1; 4, 21) that are upregulated in several different models of muscle atrophy (30). In muscle cell cultures, dexamethasone increased MAFbx/atrogin-1 and MuRF1 mRNA expression (50, 58, 68), the induction of which was shown in part to be related to the influence of activated Foxo transcription factors (51, 58). Indeed, it has recently been reported that the MuRF1 promotor is a direct target of activated glucocorticoid receptors (GR) and that the promoter is synergistically activated by Foxo1 and GR (62). In the present study, It was thus surprising to note therefore that significant increments in MuRF1 mRNA abundances were 1) significantly less and 2) delayed, compared with that of MAFbx (see Fig. 6). Similar findings were reported in myotubes exposed to dexamethasone (50). A recent study in the gastrocnemius muscle of rats given dexamethasone for 13 days also reported higher expression of MAFbx compared with MuRF1 mRNA, but at a single time point (66). The provision of high-dose dexamethasone to mice, however, revealed similar increments in MAFbx and MuRF1 mRNA in the gastrocnemius at a single time point (4 days) (19). Similar increments in MAFbx mRNA were reported in the rabbit diaphragm following supratherapeutic CS administration at 1, 2, and 3 days (52). Whether animal species and/or muscle tested account for differences across the studies is unclear. Nevertheless, our data in vivo and that of the literature highlight the potent influences of CS on MAFbx expression. However, how CS specifically regulates MAFbx expression and the relative importance of the two atrogenes in this model needs still to be elucidated. One cannot rule out redundancy of the various atrogenes in the response to CS as well.
Integration of Time Course Results
Disordered muscle protein turnover and muscle atrophy under conditions of metabolic stress is a dynamic process. This highlights the importance of a time-series approach to assess biochemical responses to a catabolic provocation. Distinct time-series differences were noted in several parameters assessed in our study.
First, the decrement in body weight was sustained with the relatively steep slope of decline the same for all groups across common time frames. The decline in body weight however tended to diminish from day 5. This suggests a very early impact of CS with possibly some “adaptive metabolic brakes” in play from day 5. We postulate that attenuation in impaired muscle protein translation initiation may be one of several possible factors over time. Indeed the significant reduction in key effectors of muscle protein synthesis influenced by CS (e.g., p70S6K) appears to be unaffected following 5 days of CS administration, suggesting some recovery of muscle protein synthetic function at this later time point (37).
We also observed an apparent discordance between early body weight loss and the expression of proteolytic markers (muscle-specific ubiquitin E3 ligases and Foxo transcription factors) and decrements in muscle mass at later temporal time points. Of interest, Auclair et al. (1) also reported similar findings in a time-series study over 7 days in rats given CS. For example, body weight decreased significantly after 2 days of steroid treatment, while EDL mass was reduced only by day 4. Further, markers of the ubiquitin-proteasome system (different from our own) increased differently across the first 3 days, despite increased proteolysis by day 1. Thus both the study by Auclair et al. (1) and our own are complex to interpret, but they highlight intriguing questions related to the dynamic process induced by CS that demand further study. We speculate that several different mechanisms may underlie the early reduction in body weight and discordance with proteolytic markers and muscle morphometry in our study. Loss of body weight without loss of muscle mass has occurred because of early reduction in body fat compared with muscle tissue (e.g., 23). Other proteolytic systems may also have been activated earlier. Recently, emphasis has been placed on the important role of the lysosomal pathways in muscle proteolysis (67). It is of interest, therefore, that a gene array study of rat gastrocnemius muscle after 3 days of CS administration revealed cathepsin L as a significant early mediator of proteolysis, and not markers of the ubiquitin proteasome system (30). A phase lag between a proteolytic stimulus and sufficient muscle protein breakdown may also have delayed a statistically significant decrement in muscle mass. Reduced muscle P-Akt levels were observed early in the present study, with decreased IGF-1 mRNA abundance by day 3. These changes would be expected to impact on both synthetic and degradation arms of protein turnover. The increment in Foxo1 and -3a muscle mRNA abundances only by day 7 however suggests that dephosphorylated Akt resulted in the activation of existing Foxo transcription factors to induce atrogene expression. While the mechanisms stimulating late Foxo1 and -3a mRNA abundances and their biochemical influences later in the course of CS exposure are not known, we speculate that replenishment of atrogene proteins would be required because of 1) the need to process/remove disassembled muscle proteins via the ubiquitin-proteasome system [the initial process of myofibrillar disassembly could be mediated by the caspase and/or calpain pathways (14, 20, 64)]; and 2) an ongoing CS-induced proteolytic state, with ATP-dependent proteolysis of myofibrillar proteins now fully activated.
The level of expression of MAFbx and its timing differed from MuRF1. The specific pathobiological roles and consequences of the two atrogenes in this model remain to be determined. Our data highlight the complexity and dynamic nature of several interacting systems and pathways in vivo. Indeed, this complexity in vivo underlies the very rationale for time-series studies, and provides intriguing questions for future studies.
In conclusion, this study demonstrates in vivo muscle expression of important signaling factors involved in CS-induced muscle atrophy in a time-series design. In addition, the dose of CS employed was well within dosing regimens used in clinical practice and thus offers insights that may be clinically applicable. Understanding the dynamic responses to catabolic challenges is clearly important in understanding the mechanisms underlying muscle atrophy and the development of therapeutic measures to offset this. As speculated above, our time-series data provide strong inference that multiple different proteolytic pathways are likely involved in CS-induced muscle atrophy. Their involvement, as well as their dynamic and complex interactions with ATP-dependent proteolysis over time, certainly needs further study. In addition, the exact roles and possible redundancy of MAFbx and MuRF1, as well as Foxo1 and Foxo3a, merit further intense evaluation.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant HL-071227.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Auclair D, Garrel DR, Zerouala AC, Ferland LH. Activation of the ubiquitin pathway in rat skeletal muscle by catabolic doses of glucocorticoids. Am J Physiol Cell Physiol 272: C1007–C1016, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Bach PB, Brown C, Gelfand SE, McCrory DC. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med 134: 600–620, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bernard S, LeBlanc P, Whittom F, Carrier G, Jobin J, Belleau R, Maltais F. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: 629–634, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Bodine SC, Latres E, Baumhueter S, Lai VKL, Nunez L, Clarke B, Poueymirou W, Panaro F, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bolton CF. Sepsis and the systemic inflammatory response syndrome: neuromuscular manifestations. Crit Care Med 24: 1408–1416, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 7. Callahan C, Dittus R, Katz B. Oral corticosteroid therapy for patients with stable chronic obstructive pulmonary disease: a meta-analysis. Ann Intern Med 114: 216–223, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Chrysis D, Underwood LE. Regulation of components of the ubiquitin system by insulin-like growth factor-I and in skeletal muscle of rats made catabolic with dexamethasone. Endocrinology 140: 5635–5641, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Combaret L, Taillandier D, Dardevet D, Béchet D, Rallière C, Claustre A, Grizard J, Attaix D. Glucocorticoids regulate mRNA levels for subunits of the 19S regulatory complex of the 26S proteasome in fast-twitch skeletal muscles. Biochem J 378: 239–246, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czerwinski SM, Zak R, Kurowski TT, Falduto MT, Hickson RC. Myosin heavy chain turnover and glucocorticoid deterrence by exercise in muscle. J Appl Physiol 67: 2311–2315, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Decramer M, deBock V, Dom R. Functional and histologic picture of steroid-induced myopathy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 153: 195819–195864, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med 150: 11–16, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Decramer M, Stas KJ. Corticosteroid-induced myopathy involving respiratory muscles in patients with chronic obstructive pulmonary disease or asthma. Am Rev Respir Dis 146: 800–822, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Du J, Wang X, Miereles C, Bailey JL, DeBigare R, Zheng B, Price R, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dumas JF, Simard G, Roussel D, Douay O, Foussard F, Malthiery Y, Ritz P. Mitochondrial energy metabolism in a model of undernutrition induced by dexamethasone. Br J Nutr 90: 969–977, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fang CH, Li B, James JH, Yahya A, Kadeer N, Guo X, Xiao C, Supp DM, Kagan RJ, Hasselgren PO, Sheriff S. GSK-3β activity is increased in skeletal muscle after burn injury in rats. Am J Physiol Regul Integr Comp Physiol 293: R1545–R1551, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Fournier M, Huang ZS, Li H, Da X, Cercek B, Lewis M. Insulin-like growth factor I prevents corticosteroid-induced diaphragm muscle atrophy in emphysematous hamsters. Am J Physiol Regul Integr Comp Physiol 285: R34–R43, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Gayan-Ramirez G, Vanderhoydone F, Verhoeven G, Decramer M. Acute treatment with corticosteroids decreases IGF-I and IGF-2 expression in the rat diaphragm and gastrocnemius. Am J Respir Crit Care Med 159: 283–289, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, Ketelslegers JM, Thissen JP. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 148: 452–460, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 86: 19–35, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gross DN, van den Heuvel APJ, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene 27: 2320–2336, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Gutman R, Choshniak I, Kronfeld-Schor N. Defending body mass during food restriction in Acomys russatus: a desert rodent that does not store food. Am J Physiol Regul Integr Comp Physiol 290: R881–R891, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Hasselgren PO. Glucocorticoids and muscle catabolism. Current Opin Clin Nutr Metab Care 2: 201–205, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Hoffman EP, Nader GA. Balancing muscle hypertrophy and atrophy. Nature Med 10: 584–485, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci 120: 2479–2487, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Kanda F, Takatani K, Okuda S, Matsushita T, Chihara K. Preventive effects of insulin-like growth factor-I on steroid-induced muscle atrophy. Muscle Nerve 22: 213–217, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Kelly FJ, McGrath JA, Goldspink DF, Cullen MJ. A morphological biochemical study on the actions of corticosteroids on rat skeletal muscle. Muscle Nerve 9: 1–10, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Kim KY, Baek A, Hwang JE, Choi YA, Jeong J, Lee MS, Cho DH, Lim JS, Kim KI, Yang Y. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res 69: 4018–4026, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Komamura K, Shirotani-Ikejima H, Tatsumi R, Tsujita-Kuroda Y, Kitakaze M, Miyatake K, Sunagawa K, Miyata T. Differential gene expression in rat skeletal and heart muscle in glucocorticoid-induce myopathy: analysis by microarray. Cardiovasc Drug Ther 17: 303–310, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Kritsch KR, Murali S, Adamo ML, Ney DM. Dexamethasone decreases serum and liver IGF-I and maintains liver IGF-1 mRNA in parenterally fed rats. Am J Physiol Regul Integr Comp Physiol 282: R528–R536, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280: 2737–2744, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18: 39–51, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Lewis MI, Li H, Huang ZS, Biring MS, Cercek B, Fournier M. Influence of varying degrees of malnutrition on IGF-I expression in the rat diaphragm. J Appl Physiol 95: 555–562, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Lewis MI, Monn SA, Sieck GC. Effect of corticosteroids on diaphragm fatigue, SDH activity, and muscle fiber size. J Appl Physiol 72: 293–301, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Li BG, Hasselgren PO, Fang CH. Insulin-like growth factor-I inhibits dexamethasone-induced proteolysis in cultured L6 myotubes through PI3K/Akt/GSK-3β and PI3K/Akt/mTOR-dependent mechanisms. Int J Biochem Cell Biol 37: 2207–2216, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Long W, Wei L, Barrett EJ. Dexamethasone inhibits the stimulation of muscle protein synthesis and PHAS-I and p70 S6-kinase phosphorylation. Am J Physiol Endocrinol Metab 280: E570–E575, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab 285: E363–E371, 2003 [DOI] [PubMed] [Google Scholar]
- 39. McEvoy C, Niewoehner D. Adverse effects of corticosteroid therapy for COPD. Chest 111: 732–743, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Mitch WE, Goldberg AL. Mechanisms of muscle wasting. N Engl J Med 335: 1897–1905, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Moore BJ, Miller MJ, Feldman HA, Reid MB. Diaphragm atrophy and weakness in cortisone-treated rats. J Appl Physiol 67: 2420–2426, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Nair KS. Muscle protein turnover: methodological issues and the effect of aging. J Gerontol 50A: 107–112, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, Anderson P, Morgan NA. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 340: 1941–1947, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Nystrom GJ, Lang CH. Sepsis and AMPK activation by AICAR differentially regulate FoxO-1, -3 and -4 mRNA in striated muscle. Int J Clin Exp Med 1: 50–63, 2008 [PMC free article] [PubMed] [Google Scholar]
- 45. Ohyama T, Sato M, Niimi M, Hizuka N, Takahara J. Effects of short and long-term dexamethasone treatment on growth and growth hormone (GH)-releasing hormone (GRH)-GH-insulin-like growth factor-I axis in conscious rats. Endocr J 44: 827–835, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Pardo PS, Lopez MA, Boriek AM. FOXO transcription factors are mechanosensitive and their regulation is altered with aging in the respiratory pump. Am J Physiol Cell Physiol 294: C1056–C1066, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Pauwels RA, Buist AS, Calverley PMA, Jenkins CR, Hurd SS, for the Scientific Committee GOLD Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Crit Care Med 163: 1256–1276, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Rannels SR, Jefferson LS. Effects of glucocorticoids on muscle protein turnover in perfused rat hemicorpus. Am J Physiol Endocrinol Metab 238: E564–E572, 1980 [DOI] [PubMed] [Google Scholar]
- 49. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 353–386, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287: E591–E601, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sassoon CSH, Zhu E, Pham TP, Nelson RS, Fang L, Baker MJ, Caiozzo VJ. Acute effects of high-dose methylprednisolone on diaphragm muscle function. Muscle Nerve 38: 1161–1172, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Schakman O, Gilson H, de Coninck V, Lause P, Verniers J, Havaux X, Ketelslegers JM, Thissen JP. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology 146: 1789–1797, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Schakman O, Kalista S, Bertrand L, Lause P, Verniers J, Ketelslegers LM, Thissen JP. Role of Akt/GSK-3beta/beta-catenin transduction pathway in the muscle anti-atrophy action of insulin-like growth factor-I in glucocorticoid-treated rats. Endocrinology 149: 3900–3908, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Seene T, Alev K. Effect of glucocorticoids on the turnover rate of actin and myosin heavy and light chains on different types of skeletal muscle fibers. J Steroid Biochem 22: 761–771, 1985 [DOI] [PubMed] [Google Scholar]
- 56. Shah OJ, Anthony JC, Kimball SR, Jefferson LS. Glucocorticoids oppose translational control by leucine in skeletal muscle. Am J Physiol Endocrinol Metab 279: E1185–E1190, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Shah OJ, Kimball SR, Jefferson LS. Acute attenuation of translation initiation and protein synthesis by glucocorticoids in skeletal muscle. Am J Physiol Endocrinol Metab 278: E76–E82, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 154: 407–412, 1986 [DOI] [PubMed] [Google Scholar]
- 60. Topp KS, Painter PL, Walcott S, Krasnoff JB, Adey D, Sakkas GK, Taylor J, McCormick K, TeNyenhuis M, Iofina M, Tomlanovich S, Stock P. Alterations in skeletal muscle structures are minimized with steroid withdrawal after renal transplantation. Transplantation 76: 667–673, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Van der Heide LP, Hoekman MFM, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 380: 297–309, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab 295: E785–E797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang L, Luo GJ, Wang JJ, Hasselgren PO. Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock 10: 298–306, 1998 [DOI] [PubMed] [Google Scholar]
- 64. Williams AB, DeCourten-Myers GM, Fisher JE, Luo G, Sun X, Hasselgren PO. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J 13: 1435–1443, 1999 [DOI] [PubMed] [Google Scholar]
- 65. Wing SS, Goldberg AL. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol Endocrinol Metab 264: E668–E676, 1993 [DOI] [PubMed] [Google Scholar]
- 66. Yin HN, Chai JK, Yu YM, Shen CA, Wu YQ, Yao YM, Liu H, Liang LM, Tompkins RG, Sheng ZY. Regulation of signaling pathways downstream of IGF-I/insulin by androgen in skeletal muscle of glucocorticoid-treated rats. Trauma 66: 1083–1090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6: 472–483, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Zhao W, Qin W, Pan J, Wu Y, Bauman WA, Cardozo C. Dependence of dexamethasone-induced Akt/FOXO1 signaling, upregulation of MAFbx, and protein catabolism upon the glucocorticoid receptor. Biochem Biophys Res Commun 378: 668–672, 2009. [DOI] [PubMed] [Google Scholar]