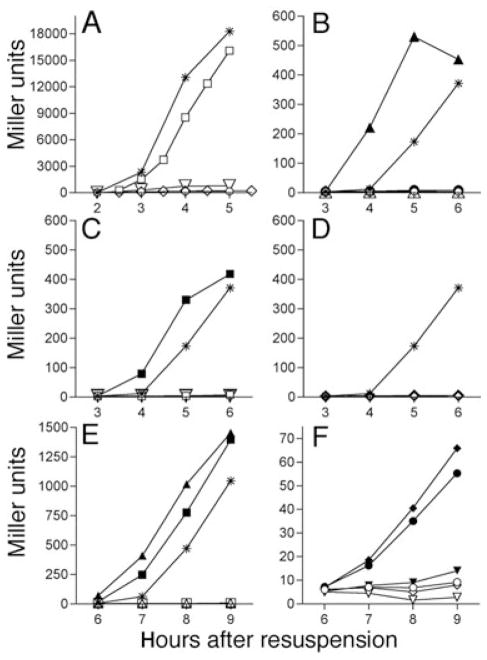
Fig. 4.
Effect of various spo mutations on σG and σK activity. Strains were induced to sporulate via resuspension with samples removed and β-galactosidase activity measured at hourly intervals.
A. Activity of σG-dependent sspB-lacZ at 37°C. Wild type (*; KP6); spoIIQ (○ KP950); spoIVB (□; KP957); spoIIIAG-H (◇; KP952); spoIIP (▽; KP951).
B–D. Activity of the σK-dependent cotD–lacZ fusion at 37°C.
B. Wild type (*; KP946), spoIIIG (△; KP947), spoIIIG bofA (▲; KP948), spoIIQ (○; KP953), spoIIQ bofA (●; KP955).
C. Wild type (*; KP946), spoIIP (▽; KP958), spoIIP bofA (▼; KP959), spoIVB (□; KP954); spoIVB bofA (■; KP956).
D. Wild type (*; KP946), spoIIIAG-H (◇; KP962); spoIIIAG-H bofA (◆; KP963), spoIVB (□; KP954); spoIVB bofA (■; KP956).
E–F. Activity of the σK-dependent cotD–lacZ fusion at 30°C.
E. Wild type (*; KP946), spoIIIG (△; KP947), spoIIIG bofA (▲; KP948), spoIVB (□; KP954); spoIVB bofA (■; KP956).
F. spoIIQ (○; KP953), spoIIQ bofA (●; KP955), spoIIP (▽; KP958), spoIIP bofA (▼; KP959), spoIIIAA-H (◇; KP960); spoIIIAA-H bofA (◆; KP961). Identical results were obtained with other engulfment mutants (spoIID or spoIIM).