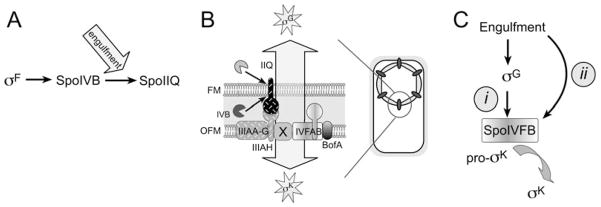
Fig. 7.
Summary and model of engulfment regulated events.
A. SpoIIQ degradation depends on the SpoIVB serine protease produced before engulfment by σF, but commences after engulfment, which must regulate either protease activity or access to its substrate.
B. The forespore protein SpoIIQ recruits the mother cell proteins SpoIIIAH and SpoIVFB to the outer forespore membrane (OFM) thereby assembling a protein complex containing proteins required for the engulfment-dependent activation of transcription in both cells (arrow). We propose that this synapse-like complex serves as a dedicated location for cell-cell communication.
C. We speculate that σK activity is governed by two checkpoints, the previously described σG checkpoint (i), in which the production of high levels of SpoIVB by σG relieves BofA-mediated inhibition of SpoIVFB and indirectly couples σK to engulfment, and a separate checkpoint (ii) that directly couples σK to engulfment, which could be comprised of a second inhibitory pathway or an activator.