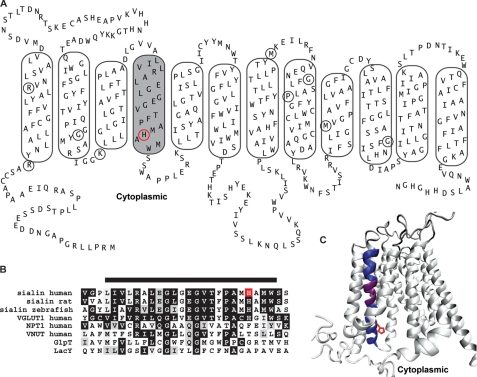
FIGURE 1.
Conserved α-helical structure is predicted to form TM4 in sialin. A, PredictProtein-derived topology model for rat and human sialin sequences with manual adjustments. Putative TMs are outlined with TM4 shaded. Circled residues correspond to those mutated in the lysosomal free sialic acid storage disorders. B, alignment of TM4 and the surrounding residues in sialin of indicated species and human isoforms of VGLUT1, the Na+-dependent phosphate transporter, and VNUT, and the bacterial transporters GlpT and LacY. Bar above the sequence indicates residues predicted to form TM4. C, three-dimensional model of sialin based on crystal structure of GlpT. TM4 is colored dark blue with the conserved GXXXG motif in purple. His-183, a TM4 residue affected by a disease-associated mutation (H183R), is depicted in red in A (circle), B (box), and C (side chain).