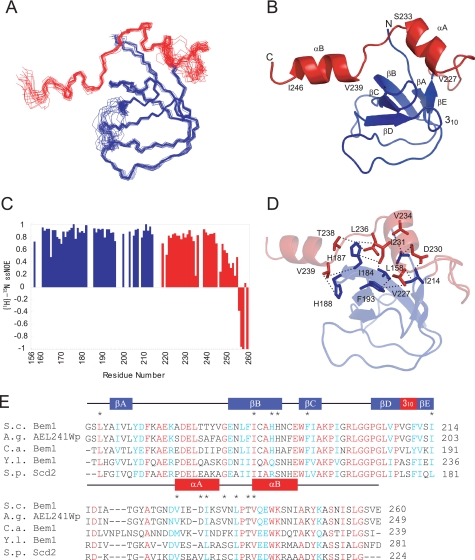
FIGURE 2.
Solution structure of Bem1 SH3b-CI. A, overlay of the backbone atoms (N, Cα, and C′) of the 20 lowest energy structures of Bem1 SH3b-CI. The SH3b and CI moieties are shown in blue and red, respectively. B, ribbon diagram of Bem1 SH3b-CI. The C-terminal flexible regions (248–260) are omitted. C, main chain dynamics of Bem1 SH3b-CI. The {1H}-15N heteronuclear NOE intensities are plotted against the residue number of Bem1 SH3b-CI. D, intramolecular interactions in Bem1 SH3b-CI. The residues involved in the hydrophobic interactions between SH3b and CI are shown in stick models. Bem1 SH3b and CI are shown in blue and red, respectively. E, sequence alignment among Bem1 SH3b-CI homologs. The secondary structural elements are indicated above the alignment. The conserved residues are marked in red, whereas type-conserved residues are marked in blue. The residues involved in the interactions between Bem1 SH3b and CI are marked with asterisks above the alignment. The atomic coordinates of SH3b-CI were deposited in the Protein Data Bank (PDB code: 2RQV).