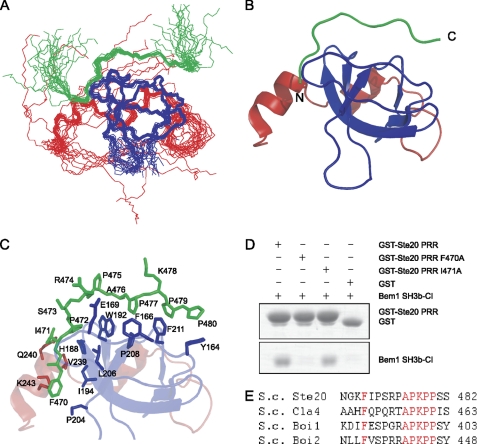
FIGURE 3.
Solution structure of Bem1 SH3b-CI and Ste20 PRR complex. A, overlay of the backbone atoms (N, Cα, and C′) of the 20 lowest energy structures of Bem1 SH3b-CI in complex with Ste20 PRR. Bem1 SH3b, Bem1 CI, and Ste20 PRR are shown in blue, red, and green, respectively. B, ribbon diagram of Bem1 SH3b-CI complexed with Ste20 PRR. Flexible regions in the N and C termini are omitted. C, interface between Bem1 SH3b-CI and Ste20 PRR. Bem1 SH3b, Bem1 CI, and Ste20 PRR are shown in blue, red, and green, respectively. The residues involved in interactions between Bem1 SH3b-CI and Ste20 PRR are shown in stick models. D, mutational analysis of Ste20 PRR. The GST-Ste20 PRR, GST-Ste20 PRR F470A, GST-Ste20 PRR I471A, or GST was incubated with Bem1 SH3b-CI. The proteins pulled down with glutathione-Sepharose 4B were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue. E, sequence alignment of Bem1 SH3b-CI-binding proteins. The conserved residues are marked in red. The atomic coordinates of SH3b-CI complexed with Ste20 PRR were deposited in the Protein Data Bank (PDB code: 2RQW).