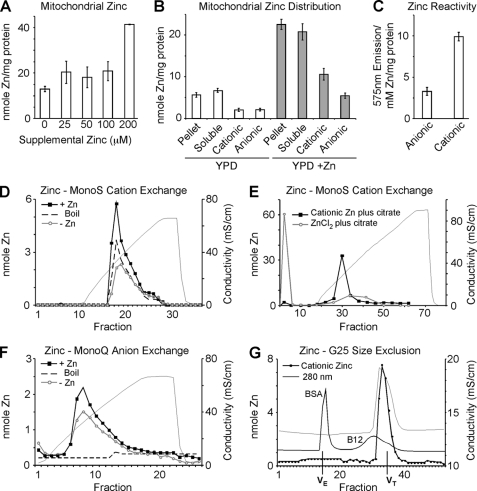
FIGURE 1.
Mitochondrial zinc pools. Yeast cultures were grown in yeast peptone 1% dextrose (YP1D) medium and supplemented with 0–200 μm ZnCl2. Mitochondria were isolated from cultures in triplicate and analyzed for zinc either from total or submitochondrial fractions by ICP-OES. Panel A, titration of supplemental zinc to cell cultures results in expansion of mitochondrial zinc. Panel B, soluble and pellet fractions were isolated from 0.5 mg of mitochondria by sonication and centrifugation. Ion exchange using either CM-52 or DEAE-52 cellulose was used to separate cationic and anionic fractions respectively. Panel C, using the Zn-dependent fluorophore Rhodzin-3, reactive zinc was assessed in mitochondrial lysate from 0.1 mg of mitochondria depleted of either anionic or cationic Zn pools (post DEAE-52 or CM-52 binding, respectively; n = 4). Panels D–F, MonoS and MonoQ ion exchange chromatography. Mitochondria were isolated from YP1D yeast cultures grown with 0.2 mm Zn (+Zn) or without (−Zn, Endogenous) and samples (equivalent to 0.5 mg of protein) were sonicated, clarified, and boiled where indicated. Panel D, fractionation of soluble lysate using MonoS shows a single cationic zinc peak that expands in response to zinc supplementation and is resistant to boiling. Panel E, either free Zn(II) in the form of ZnCl2 or previously purified mitochondrial cationic Zn were mixed with a 10-fold excess of citrate and examined for the ability to bind to MonoS cation exchange resin. Panel F, fractionation of soluble lysate using MonoQ anion exchange reveals a broad peak that expands modestly in response to zinc supplementation, yet is not resistant to boiling. Panel G, gel filtration of the cationic zinc fraction isolated by MonoS elutes on a G25 size exclusion column in a volume corresponding to salt. 280 nm absorbance for both bovine serum albumin (BSA) and Vitamin B12 (B12) are shown. Volume exclusion (VE) and volume total (VT) are marked.