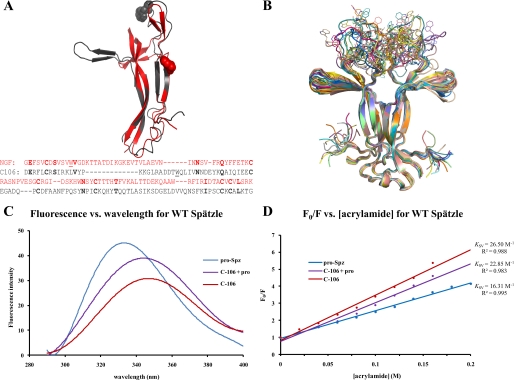
FIGURE 1.
Proteolytic activation of Spätzle induces a conformational change. A, structural and sequence alignment of nerve growth factor (residues 131–236, gray) and Spätzle C-106 (arbitrary sequence numbers because of N-terminal splice variant) (see “Experimental Procedures”). Tryptophan residues are illustrated as spheres, and the structures have a C-α backbone root means square deviation of 2.72 Å. In the sequence alignment, identical residues are in bold, and the tryptophans are underlined. B, C-106 dimer structure (18). The tryptophan loop is shown in 20 possible conformations selected on the basis of energy minimization. C, fluorescence spectroscopy of Spätzle (see “Experimental Procedures”). Red shifting of the spectra shows that the tryptophan residue is increasingly exposed as the prodomain is cleaved but remains noncovalently attached (C-106 + pro) and then most exposed once the prodomain is completely removed (C-106). D, quenching of the fluorescence signal using acrylamide. This allows for the calculation of the Stern–Volmer constant (Ksv), which gives a quantitative indication of the level of exposure of the tryptophan residue; the higher Ksv values indicate increased exposure of the residue.