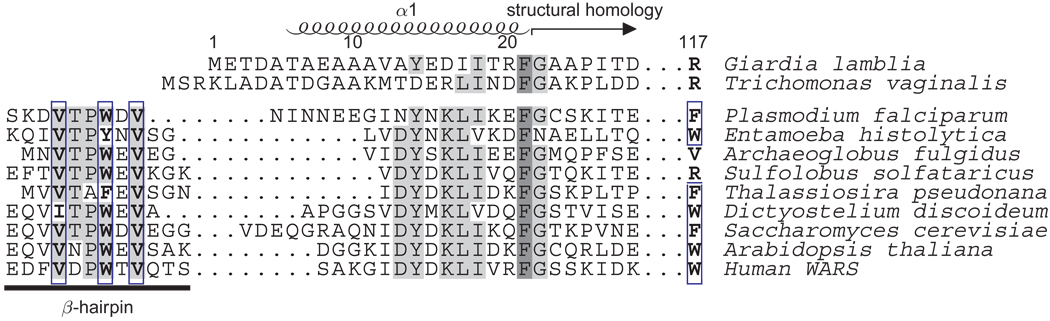
Figure 1. Sequence alignment of the G. lamblia TrpRS N-terminus with other eukaryotic and archaeal sequences.
The Giardia and human TrpRS are structurally homologous beginning at residue 20. In the Giardia structure, residues N-terminal to this point form part of an initial α-helix comprising residues 6–22. In the human TrpRS these residues have been observed instead to form a β-hairpin involved in ATP binding. A triad Val-Trp-Val of hydrophobic residues, shown boxed in the figure, stabilizes association of the hairpin with a Trp residue at the positions equivalent to Arg 117 in the Giardia sequence, and with a highly conserved Phe residue equivalent to Phe 301 in the Giardia sequence. This hydrophobic triad and the corresponding tryptophan are recognizably present in most eukaryotic TrpRS sequences, but are missing from some protozoan homologs.