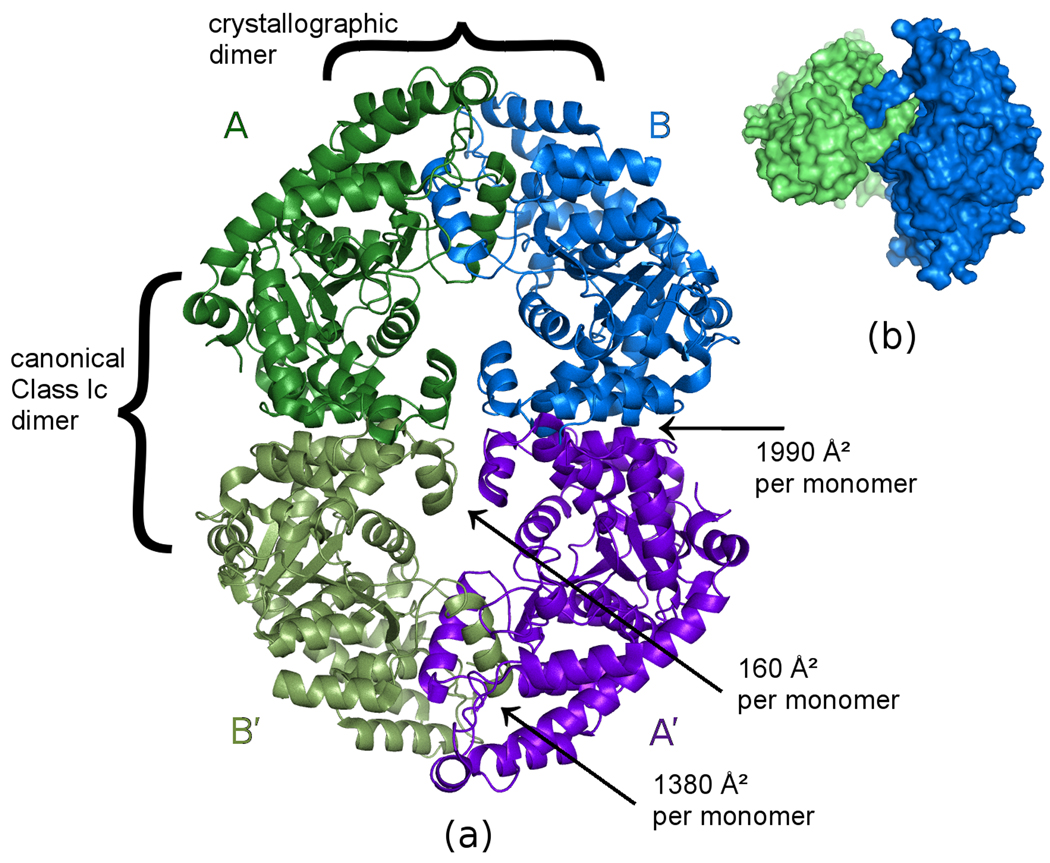
Figure 2. Crystallographically observed tetramer of G. lamblia TrpRS.
(a) The two monomers in the upper half of the figure are crystallographically independent. The view is along the crystallographic 2-fold axis relating the monomers in the upper half of the figure to those in the lower half. The pair of monomers on the left (dark green, light green) and the pair monomers on the right (light blue, dark blue) each form the canonical dimer previously observed for all class Ic aminoacyl-tRNA synthetases. The dimer formed by the two crystallographically independent monomers (dark green, light blue) buries 1380 Å2 of accessible surface on the N-terminus of each monomer due to the interlocked α1 helices, plus an additional 160 Å2 per monomer due to reciprocal interaction of the α11 helices. Buried surface areas were calculated using PISA (Krissinel and Henrick, 2007). (b) Surface representation of the two crystallographically independent monomers, showing how they reciprocally interdigitate to form a dimer. The view is rotated roughly 90° from the view in (a).