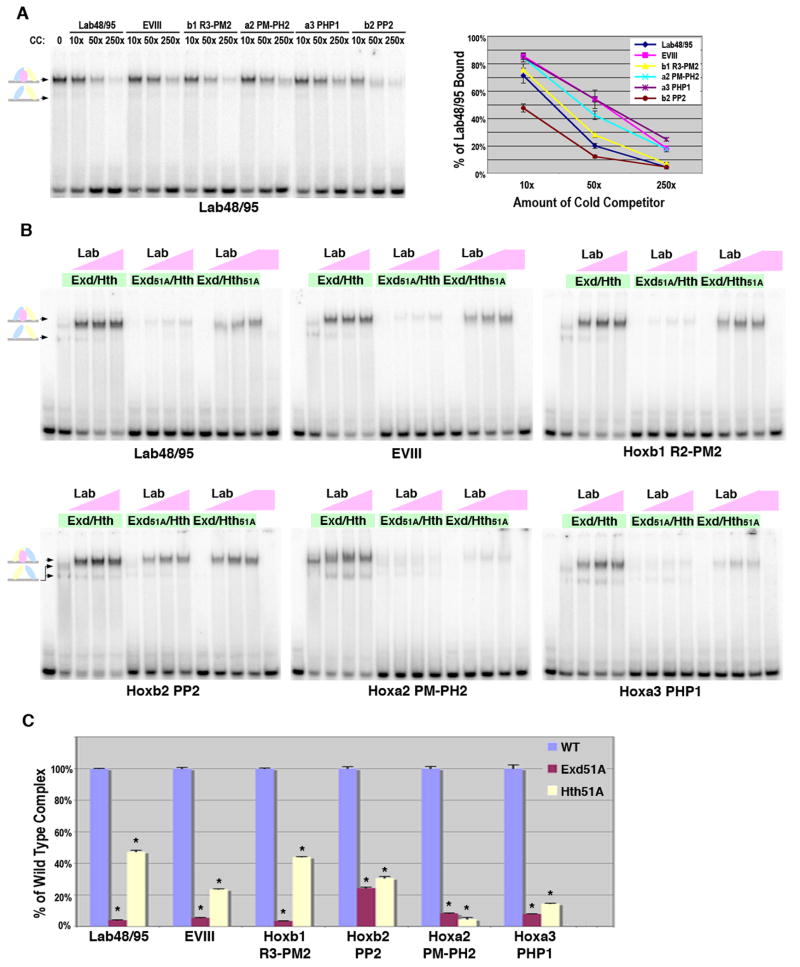
Figure 5. Comparisons between Lab-Exd-Hth binding sites.
A. DNA binding competition assays for Lab, Exd and Hth complexes on Lab48/95, EVIII, Hoxb1 R3-PM2, Hoxa2 PM-PH2, Hoxa3-PHP1, and Hoxb2-PP2 probes (see Figure 1 for sequences). Labeled Lab48/95 probe was bound with a constant amount of Exd/Hth (75 × 10-9 M) and Lab (110 × 10-9 M). Different amounts of competitor were added as indicated. Schematics at left denote color-coded complexes (Exd, blue; Hth, yellow; Lab, pink). The amount of probe bound in absence of competitor was assigned 100% binding and the graph (at right) depicts the average amount of probe bound in presence of competitor from three different experiments with standard error noted. B. Comparative EMSAs using wild type and mutant Exd/Hth heterodimers with Lab on Lab48/95, EVIII, Hoxb1 R3-PM2, Hoxa2 PM-PH2, Hoxa3-PHP1, and Hoxb2-PP2 probes. Equimolar amounts of Exd/Hth, Exd51A/Hth, and Exd/Hth51A proteins (30 × 10-9 M) were used with three different amounts of Lab (12 × 10-9 M, 36 × 10-9 M, or 110 × 10-9 M). C. Assessing the dependence of Lab complex formation on Exd and Hth binding to Lab48/95, EVIII, Hoxb1 R3-PM2, Hoxa2 PM-PH2, Hoxa3-PHP1, and Hoxb2-PP2. Comparative DNA binding assays were performed in triplicate using equimolar amounts of Exd/Hth, Exd51A/Hth, and Exd/Hth51A proteins (30 × 10-9 M) and Lab (110 × 10-9 M). The amount of probe bound by Abd-A and wild type Exd/Hth was assigned to 100% for each probe tested (blue bar). The amount of probe bound by Exd51A/Hth (red bar) and Exd/Hth51A (yellow bar) compared to wild type was then determined. * denotes a significant difference from wild type binding (p-value < 0.001).