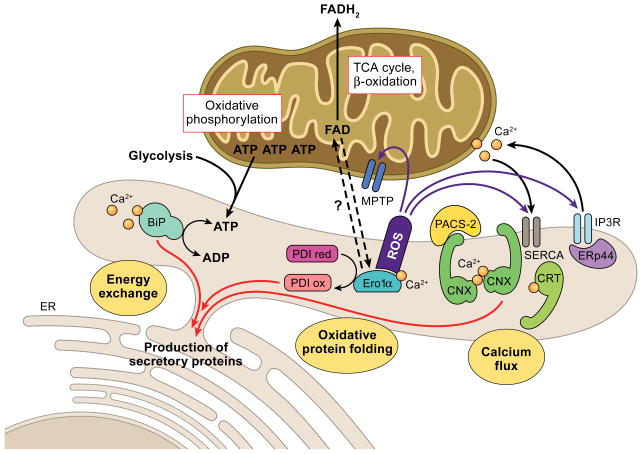
Figure 1.
Three major metabolic exchanges between the ER and mitochondria impact on ER chaperone systems that catalyze the production of secretory proteins. 1. Energy exchange. GRP78/BiP requires ATP that is predominantly supplied by mitochondria, and to a lesser extent from glycolysis. GRP78/BiP also depends on ER calcium. 2. Oxidative protein folding. PDI forms disulfide bonds with the help of its recharger Ero1α, which can bind calcium and FAD derived from mitochondrial metabolism. Import proteins of FAD into the ER are currently unknown in mammalian systems. ROS produced by Ero1α could directly impact on the mitochondrial permeability transition pore (MPTP), SERCA and the IP3R. 3. Calcium flux. The calnexin/calreticulin folding cycle depends on ATP and ER calcium. Calnexin and calreticulin buffer free calcium within the ER, but also associate with SERCA to modulate calcium import. The localization of calnexin to the MAM and hence potentially its interaction with SERCA is under the control of PACS-2. The MAM-associated moiety of the oxidoreductase ERp44 interacts with the IP3R and regulates IP3R calcium release.