Fig. 6.
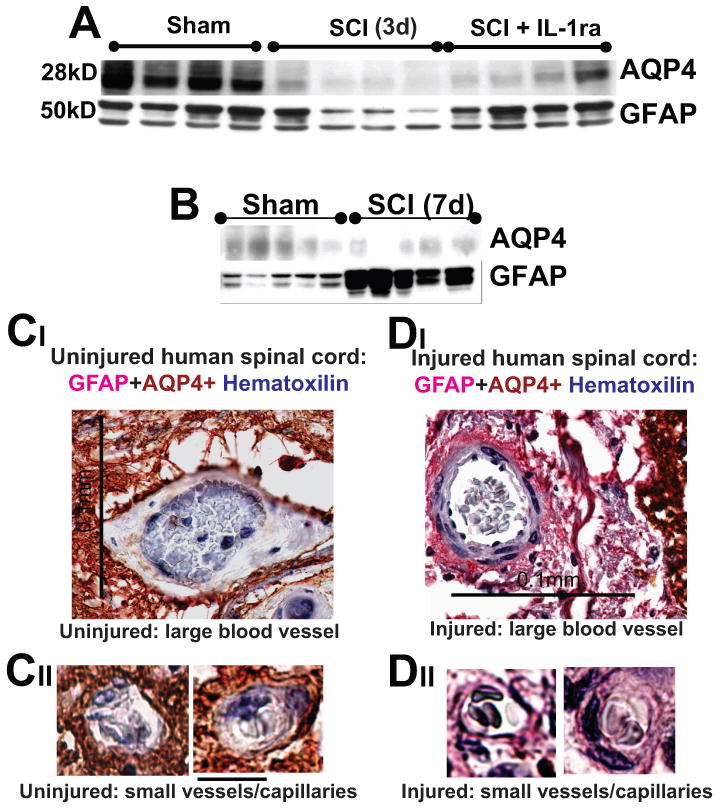
A) AQP4 and GFAP Western blots 3d after SCI at the lesion site (T10). IL1ra (750ng/ml) was administered intrathecally for 3d in a group of SCI rats (n=5), while a second group of SCI rats received vehicle intrathecally for 7d (n=4). Rats were sacrificed at 3d post-SCI and Western blots performed. IL1ra was administered via osmotic minipumps and i.th. cathaters. We have also shown that IL1ra exerted expected effects: it significantly decreased Cox-2 upregulation after SCI, or reduced IL-6 mRNAs (not shown; both Cox-2 and IL-6 are known IL-1 transcriptional targets – Touzani et al., 1999; Igwe et al., 2001). Therefore, the IL-1ra effects we observed in injured cords were mediated via specific blocking of IL-1R activation. B) AQP4 and GFAP Western blots showed that 7d after SCI, AQP4 levels were still reduced at the lesion site in moderate SCI, while GFAP levels showed significantly increased levels compared to sham-treated rats (n=5 per group), indicating replacement and activation of astrocytes early after SCI (already reported in Nesic et al., 2006). C, D) Large and small blood vessels in uninjured and injured human spinal cord (C2, 1 year after SCI, SCI patient No. 29; Guest et al., 2005). Calibration line in CI :0.1 mm; and in CIII: 10 μm. GFAP-labeled astrocytic processes devoid of AQP4 (pink) surrounded blood vessel in chronically injured cords. Erythrocytes in blood vessel were stained blue with hematoxilline.
