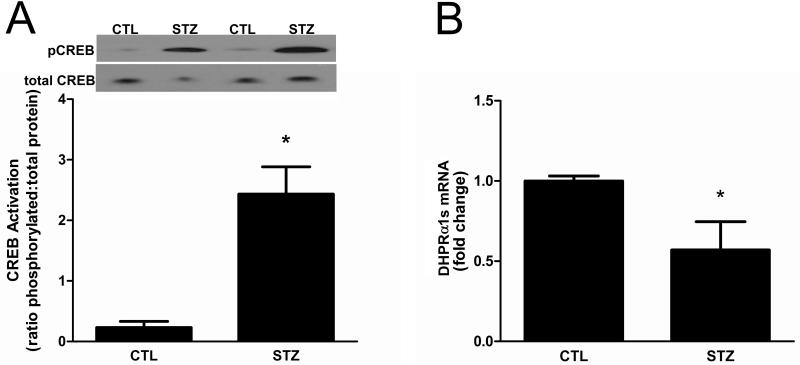
Figure 3. CREB signaling is abnormal in 21day STZ-treated rat muscle.
(A) The phosphorylation (i.e., activation) status of CREB in gastrocnemius muscles of control and 21d STZ-treated rats was examined by Western blot analysis using antibodies that detect phospho-Ser133 and total CREB. Data are expressed as the mean ratio of phosphorylated protein to total protein ± SEM. Equal protein loading and transfer were confirmed by Ponceau S staining. n=6/group, *P<0.05. (B) To evaluate CREB function, the amounts of DHPRα1s, a gene target of CREB, were measured in gastrocnemius muscles by real time RT-PCR. Data are expressed as the mean ± SEM; n=8/group, *P<0.05.