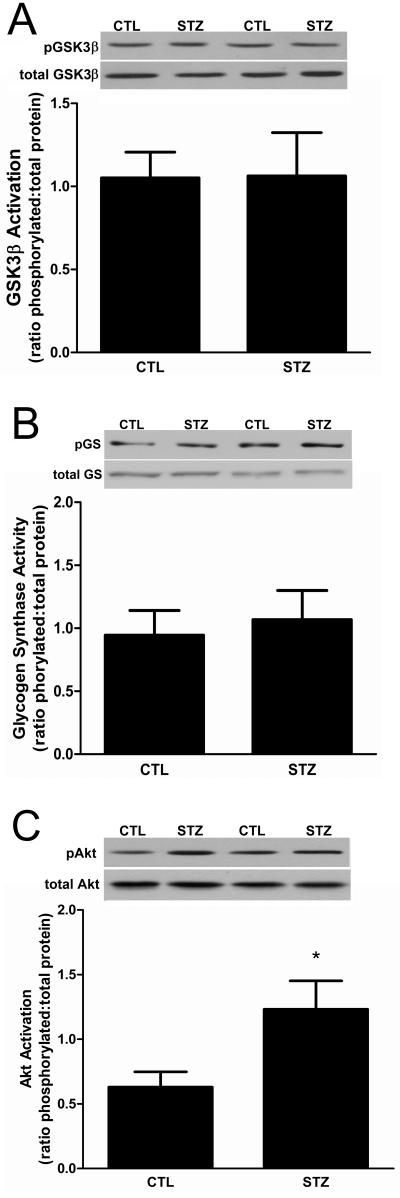
Figure 6. GSK-3β signaling is unchanged in 21day STZ-treated rat muscle.
(A) The phosphorylation (i.e., inactivation) of GSK-3β on Ser-9 in gastrocnemius muscles was examined by Western blot analysis using antibodies that detect phospho-S9 and total GSK-3β. Data are expressed as the mean ratio of phosphorylated protein to total protein ± SEM. n= 8/group; P=0.31 (B) The phosphorylation of glycogen synthase on Ser-641 also was examined as a downstream target of GSK-3β activity by Western blot analysis using antibodies that detect total and phospho-Ser-641 glycogen synthase. Data are expressed as the mean ratio of phosphorylated protein to total protein ± SEM. n= 8/group; P=0.69 (C) Western blot analysis of Akt phosphorylation was performed using antibodies that detect total and phospho-Ser-473 Akt. Data are expressed as the mean ratio of phosphorylated protein to total protein ± SEM: P<0.05. For each experiment equal protein loading and transfer were confirmed by Ponceau S staining. n=7/group.