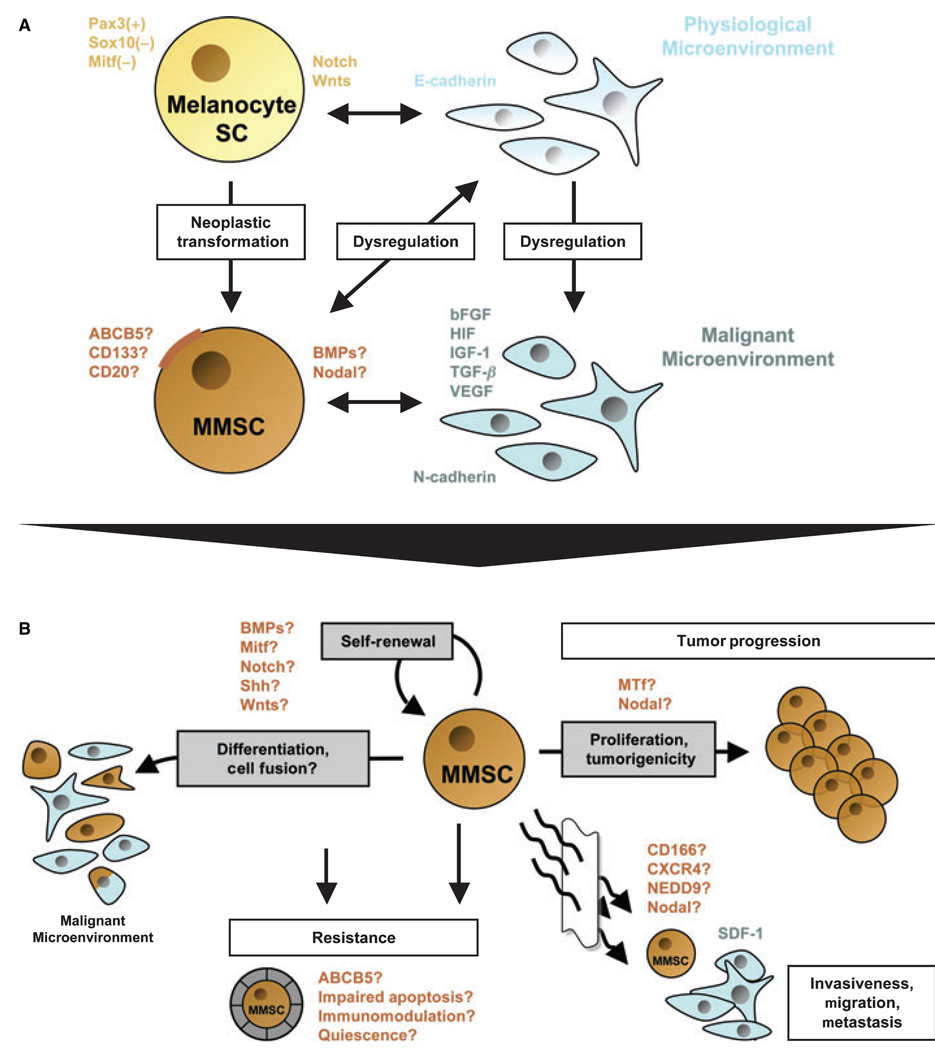
Figure 1.
Schematic model of a potential relationship between melanocyte stem cells (Melanocyte SC) and putative malignant melanoma stem cells (MMSC) in the context of distinct physiological or malignant microenvironments, and overview of defining features and functional properties ascribed to putative MMSC. (A) Physiological melanocyte stem cells are characterized by a Pax3+, Sox10− and Mitf− phenotype, which promotes immaturity and quiescence. The balance between melanocyte SC self-renewal and differentiation, quiescence and proliferation in the hair follicle is further regulated by Notch and/or Wnt signaling molecules. It is thought that the homeostasis of these lineage-restricted SC is maintained through interactions with the physiological microenvironment, mainly composed of E-cadherin-expressing keratinocytes. MMSC might arise by cumulative oncogenic mutations and/or loss of tumor suppressors from long-lived melanocyte stem cells, which already contain the machinery for indefinite self-renewal. Surface markers that have been associated with melanoma stem-like cells include ABCB5, CD133, or CD20. Moreover, morphogens such as Nodal or BMP4 may be secreted by putative MMSC populations. In addition to the neoplastic transformation of the melanocyte stem cell itself, distinct changes in the microenvironment may facilitate the formation of MMSC and subsequent MMSC-driven melanomagenesis. A dysregulated, malignant microenvironment described for melanocytic tumors is characterized by the production of large quantities of proliferative growth factors, including bFGF, HIF, IGF-1, TGF-β, and VEGF. These paracrine mediators, produced mainly by N-cadherin-expressing endothelial cells or surrounding fibroblasts, may drive MMSC proliferation and hence melanoma progression. On the other hand, MMSC might actively alter their surrounding microenvironment through secreted factors (i.e., Nodal or BMP), thus sustaining a niche hospitable to tumor initiation and growth. (B) Defining characteristics of putative MMSC populations and their potential involvement in melanoma initiation, progression, and resistance. Analogous to the cancer stem cell (CSC) definition, MMSC should exhibit (1) increased in vivo tumorigenicity, (2) in vivo self-renewal capacity, and (3) the competence to differentiate into phenotypically diverse populations, including non-tumorigenic cancer cells present in the original tumor. Regulatory signaling networks operative in physiological stem cells that have been shown to be dysregulated in malignant melanoma, such as BMP, Mitf, Notch, Shh, or Wnts, may likewise modulate the balance between self-renewal and differentiation of MMSC. Molecules that have been associated with the tumorigenic potential of malignant melanoma, and which might be involved in MMSC-driven tumor growth, include melanotransferrin (MTf) and Nodal. Transdifferentiation potential, which has been ascribed to distinct melanoma subsets, might likewise involve MMSC. In addition MMSC fusion with more differentiated, including physiological cell types, might represent a possible mechanism by which putative MMSC could mimic a physiological phenotype and function. The differentiation plasticity of MMSC may mask malignant populations from immune surveillance and could thus contribute to melanoma resistance. The mechanisms by which normal stem cells tend to be more resistant than mature cell types, including increased expression of ABC transporters, relative quiescence, immunomodulatory functions, or impairment of apoptotic pathways, may likewise contribute to the refractoriness of putative MMSC. Finally, candidate factors, such as CD166, CXCR4, or NEDD9, might also play a role in putative MMSC-driven melanoma cell invasion, migration, and metastasis, as a correlate of their known physiological and pathophysiological functions.