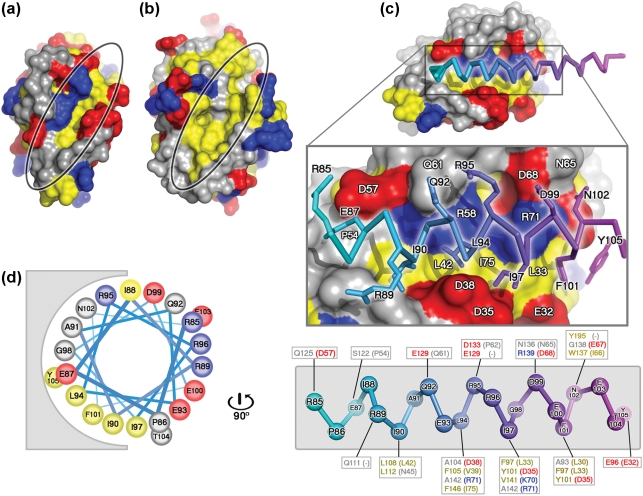
Fig. 6.
Comparison of N1 and Bcl-xL surface grooves and molecular modelling of BH3 peptides. Molecular surfaces of N1 (a) and Bcl-xL (b), with the grooves highlighted. Lys, Arg and His are coloured in blue, Asp and Glu are coloured in red and Leu, Ile, Val, Met, Tyr, Phe and Trp are coloured in yellow. All other residue types are coloured in grey. (c) Molecular surface of N1 with the Bim BH3 helix (PDB code 1PQ1) modelled into the surface groove as described in the text. The surface of N1 is coloured as described in (a). The peptide is coloured from cyan to magenta from the N to the C terminus. The middle panel shows the Bim peptide from residue 85 to 105 with contacting residues drawn in atomic detail. Residues of the peptide contacting N1 are labelled in black and residues of N1 contacting the peptide are labelled in white. The lower panel shows the Bim BH3 peptide helix skeleton coloured from cyan to magenta, with the contacting residues of Bcl-xL and their equivalents of N1 boxed (N1 residues are in parentheses), and residues coloured according to their properties, as in (a). (d) Helical wheel representation of the Bim BH3 peptide (residues 85–105), drawn rotating the peptide in (c) by 90 ° around an axis vertical to the page, showing the predominantly amphipathic nature of the BH3 helix.