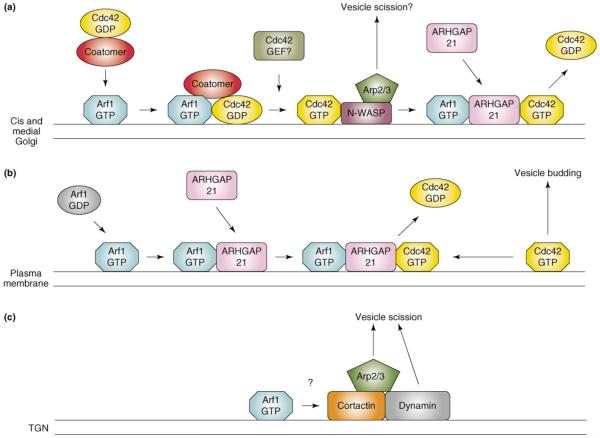
Figure 1.
Regulation of actin assembly by Arf1. (a) Arf1 acts both upstream and downstream of Cdc42 to promote vesicle formation in the Golgi. Cdc42 interacts with the γCOP subunit of the COPI coatomer complex and is brought to Golgi membranes by Arf1-mediated coatomer recruitment. The identity of the GEF that activates Golgi-associated Cdc42 is not known. Activated Cdc42 then stimulates N-WASP, which activates the Arp2/3 complex, leading to local actin assembly, and it is thought that this promotes vesicle scission. It is not known if Arf1 remains associated with the Cdc42-N-WASP complex. In addition, Arf1 also downregulates Cdc42 activity by recruiting the Cdc42 GAP, ARHGAP21, to Golgi membranes. In the absence of ARHGAP21, unregulated actin assembly leads to disruption of the Golgi complex. How the upstream and downstream functions of Arf1 are coordinated is not currently understood. (b) Arf1, Cdc42, and ARHGAP21 also cooperate at the plasma membrane to mediate clathrin-independent endocytosis. Cdc42 activation leads to the formation of endocytic vesicles containing fluid phase markers and GPI-anchored membrane proteins (vesicle budding) [22]. It is not known if this pathway also uses N-WASP to drive actin assembly. Activation of Arf1 on the plasma membrane recruits ARHGAP21, leading to downregulation of Cdc42 activity. Depletion of either Arf1 or ARHGAP21 by RNAi results in accumulation of Cdc42 in puncta at the plasma membrane [22]. (c) In the TGN, Arf1 activity promotes the assembly of a cortactin–dynamin complex at sites of vesicle formation through a mechanism that remains unknown. In a similar fashion to N-WASP, cortactin can activate the Arp2/3 complex and presumably acts in concert with dynamin to mediate vesicle scission.