Abstract
There has been extensive work to elucidate the behavioral and physiological mechanisms responsible for taste preferences of the rat but little attempt to delineate the underlying genetic architecture. Here, we exploit the FHH-Chr nBN/Mcwi consomic rat strain set to identify chromosomes carrying genes responsible for taste preferences. We screened the parental Fawn Hooded Hypertensive (FHH) and Brown Norway (BN) strains and 22 FHH-Chr nBN consomic strains, with 96-h 2-bottle tests, involving a choice between water and each of the following 16 solutions: 10 mM NaCl, 237 mM NaCl, 32 mM CaCl2, 1 mM saccharin, 100 mM NH4Cl, 32 mM sucrose, 100 mM KCl, 4% ethanol, 1 mM HCl, 10 mM monosodium glutamate, 1 mM citric acid, 32 μM quinine hydrochloride, 1% corn oil, 32 μM denatonium, 1% Polycose, and 1 μM capsaicin. Depending on the taste solution involved, between 1 and 16 chromosomes were implicated in the response. Few of these chromosomes carried genes believed to mediate taste transduction in the mouse, and many chromosomes with no candidate taste genes were revealed. The genetic architecture of taste preferences is considerably more complex than has heretofore been acknowledged.
Keywords: bitter, chemosensation, ethanol, fat taste, gene discovery, mineral taste, quantitative trait locus, salty, sour, sweet, umami
Introduction
Over the last 15 year, transduction mechanisms have been discovered for bitter, sour, salty, sweet, umami, fat, metallic, and calcium tastes (for reviews, see Kim et al. 2004; Bachmanov and Beauchamp 2007; Ramos Da Conceicao Neta et al. 2007; Boughter and Bachmanov 2008; Tordoff, Shao, et al. 2008; Mattes 2009). The impetus for many of these discoveries has come from studies revealing differences in taste solution consumption among inbred mouse strains (e.g., Lush 1991; Lush et al. 1995; Bachmanov et al. 2002; Tordoff et al. 2007b). However, the rat has long been the model of choice for studying taste and nutrition (e.g., Richter 1942–1943; Young and Greene 1953; Lindsey and Baker 2005), and the mechanisms underlying taste solution consumption in the mouse do not always apply to the rat. One example is the preference for salt: most mouse strains avoid mildly hypertonic NaCl solutions relative to water but most rat strains prefer them (Bachmanov et al. 2002; Tordoff et al. 2007b; Tordoff, Alarcon, Lawler, et al. 2008). Another is the response to sweeteners: mice choose the artificial sweetener, sucralose, in preference to water (e.g., Bachmanov, Tordoff, and Beauchamp 2001), but rats are ambivalent to it (Sclafani and Clare 2004; Bello and Hajnal 2005). Polymorphisms in the taste receptor subunit gene Tas1r3 can account for most of the variation in saccharin preference among mouse strains (Bachmanov, Li, et al. 2001; Reed et al. 2004) but little or none of the variation within or among rat strains (Lu et al. 2005). For other taste modalities, there has been insufficient comparative work to determine whether the 2 species use the same mechanisms. There is also no a priori reason to favor the mouse or the rat as the more satisfactory model of human taste preferences. Thus, genetic analyses of the rat have the potential to reveal new mechanisms responsible for taste preferences and to facilitate interpretation of the many existing findings on taste and nutrition in both rat and human.
The goal of the present work is to jump-start the study of the genetic basis of taste preferences in the rat. To this end, we have measured the responses of a consomic set of rats to 15 compounds that are commonly used as taste stimuli. Consomic rat strains, also called chromosome substitution strains, have one chromosome derived from one parental inbred strain and the other 21 chromosomes derived from a second parental inbred strain. They are a good starting point for gene discovery because differences in phenotype between a consomic strain and its recipient parent strain can be attributed to genes in the introgressed chromosome (for reviews, see Cowley, Liang, et al. 2004; Cowley, Roman, and Jacob 2004). The FHH-Chr nBN/Mcwi strain set used here involves introgression of chromosomes from the BN/SsNHsdMcwi (BN) strain onto an FHH/EurMcwi (FHH) background. The BN is one of several Brown Norway strains. It is a propitious strain for genetic studies because its genome has been sequenced (Gibbs et al. 2004; Twigger et al. 2008), and it has been extensively phenotyped in many domains, often being used as a control for other strains. The FHH strain, one of several Fawn Hooded strains, has been less well characterized. Rats of this strain have blood platelet storage-pool deficiency (Brown et al. 1996; Datta et al. 2003) and develop hypertension, renal disease, proteinuria, and hyperlipidemia as they age (Brown et al. 1996; Verseput et al. 1997). They probably have disordered brain serotonin metabolism (Gudelsky et al. 1985; Aulakh et al. 1994). The FHH-Chr nBN strain set used here was developed by Jacob and colleagues at the Medical College of Wisconsin (Mattson et al. 2007) and is commercially available from Physiogenix, Inc. These groups have collected basic biochemical, cardiac, vascular, histological, and renal data from members of the FHH-Chr nBN consomic set (PhysGen 2009), but there have been no previous reports of the behavior of these rats.
Indeed, there is almost no information about the taste preferences of the BN and FHH parental strains and very little about other related Brown Norway and Fawn Hooded strains. Brown Norway rats are considered to have low NaCl preferences (Thunhorst and Johnson 2003) but this reputation may be undeserved. It is based on comparisons of the BN/BiRijNNiaHsd and spontaneously hypertensive rat (SHR) strains (Di Nicolantonio 2004; Di Nicolantonio et al. 2004), which is unfortunate because the SHR strain is an abnormally avid NaCl consumer (Catalanotto et al. 1972; Di Nicolantonio et al. 1983; Yongue and Myers 1989) and thus a poor reference strain. Preferences for 280 mM glucose and 280 mM urea are similar in Brown Norway and SHR rats (Di Nicolantonio 2004), but once again, this is difficult to interpret because the SHR is not representative of most strains. The BN/CrJ strain had the highest intake of monosodium glutamate (MSG) out of 14 rat strains tested (Kondoh et al. 2000). Given MSG’s substantial salty taste component, it is difficult to reconcile this finding with the Brown Norway’s reputation as a sodium-avoiding strain.
With respect to Fawn Hooded rats, the FH/Wjd strain has strong preferences for ethanol and saccharin (e.g., Daoust et al. 1991; Overstreet et al. 1999, 2007; Goodwin et al. 2000; Rezvani et al. 2007), and it is insensitive to the bitter compounds cycloheximide, phenylthiocarbamide, and quinine relative to Long Evans or Wistar strains (Tobach et al. 1974; Goodwin and Amit 2000). However, the FH/Wjd strain appears to be unique in this regard; the FHH strain used here does not have such high preferences for ethanol or saccharin (Overstreet et al. 1999, reviewed in Overstreet et al. 2007), and its sensitivity to bitter compounds is untested.
We know of no other studies of the taste preferences of BN and FHH rats, apart from our own work (Tordoff, Alarcon, Lawler, et al. 2008). This involved a survey of 14 rat strains and included measurements of the responses of the BN and FHH strains to series of 4–8 concentrations of 17 taste compounds. Based on these data, we selected for use in the current study a single concentration of 15 taste compounds (or 2 concentrations of NaCl) that supported large differences in consumption between the 2 parental strains. Each of these concentrations was tested in the FHH-Chr nBN consomic strain set.
Materials and methods
Throughout the text, the term “taste solution” is used loosely, for convenience. Some of the compounds tested were not strictly solutions (e.g., corn oil emulsion) or strictly tastes (e.g., capsaicin solution), and fluid intakes in long-term 2-bottle choice tests may involve postingestive, experiential, cognitive, and nongustatory orosensory cues in addition to taste (see Discussion).
The experiment protocol was approved by the Animal Care and Use Committee of the Monell Chemical Senses Center.
Subjects and maintenance
The experiment involved male rats from the FHH strain, BN strain, and each of 22 FHH-Chr nBN/Mcwi consomic strains, with group sizes given in Table 1. BN rats were purchased from Charles River Laboratories (strain code CR-327). All the other rats were provided by Physiogenix Inc., either from a colony maintained at Hilltop Lab Animals Inc. or from the Medical College of Wisconsin. The rats were 30–44 days old when they arrived at our facility (the source and age of each rat and other details are included in online Supplementary material).
Table 1.
Group sizes, body weights, and daily water intakes of the FHH-Chr nBN consomic set of rats
| Strain | n | BWstart, g | BWend, g | Water intake, mL/d |
| FHH | 15 | 215 ± 6 | 434 ± 7 | 54 ± 2 |
| BN | 10 | 143 ± 3a | 328 ± 5a | 16 ± 0a |
| FHH-Chr 1BN | 10 | 197 ± 8 | 386 ± 6a | 29 ± 1a |
| FHH-Chr 2BN | 10 | 212 ± 13 | 399 ± 9a | 44 ± 3a |
| FHH-Chr 3BN | 10 | 201 ± 7 | 384 ± 5a | 56 ± 4 |
| FHH-Chr 4BN | 10 | 162 ± 4a | 400 ± 4a | 40 ± 3a |
| FHH-Chr 5BN | 10 | 241 ± 3b | 475 ± 8b | 56 ± 3 |
| FHH-Chr 6BN | 10c | 163 ± 9a | 373 ± 8a | 39 ± 1a |
| FHH-Chr 7BN | 10 | 257 ± 14b | 430 ± 9 | 32 ± 1a |
| FHH-Chr 8BN | 10 | 227 ± 7 | 394 ± 16a | 32 ± 1a |
| FHH-Chr 9BN | 10 | 192 ± 12a | 403 ± 8a | 48 ± 3 |
| FHH-Chr 10BN | 10 | 173 ± 8a | 423 ± 12 | 47 ± 2 |
| FHH-Chr 11BN | 10 | 143 ± 7a | 376 ± 7a | 48 ± 2 |
| FHH-Chr 12BN | 10 | 172 ± 14a | 417 ± 5 | 47 ± 2 |
| FHH-Chr 13BN | 10c | 199 ± 10 | 412 ± 11a | 48 ± 2 |
| FHH-Chr 14BN | 10 | 155 ± 9a | 369 ± 8a | 41 ± 3a |
| FHH-Chr 15BN | 10 | 169 ± 10a | 406 ± 9a | 40 ± 3a |
| FHH-Chr 16BN | 5 | 200 ± 16 | 436 ± 3 | 40 ± 4a |
| FHH-Chr 17BN | 5 | 175 ± 4a | 430 ± 9 | 41 ± 3a |
| FHH-Chr 18BN | 10 | 165 ± 14a | 383 ± 13a | 35 ± 1a |
| FHH-Chr 19BN | 6 | 196 ± 20 | 413 ± 7a | 40 ± 1a |
| FHH-Chr 20BN | 10 | 200 ± 6 | 399 ± 6a | 43 ± 4a |
| FHH-Chr XBN | 5 | 181 ± 7a | 393 ± 10a | 42 ± 1a |
| FHH-Chr YBN | 10 | 188 ± 10a | 390 ± 10a | 51 ± 2 |
BWstart = body weight at start of testing, when rats were ∼50 days old; one-way ANOVA, F23,204 = 8.51, P < 0.0001. BWend = body weight at end of testing, when rats were ∼131 days old; one-way ANOVA, F23,201 = 10.4, P < 0.0001. Water intake = daily water intake based on 17 one-bottle tests given between each 4-day 2-bottle test.
P < 0.01 less than FHH strain.
P < 0.01 greater than FHH strain.
A rat from this group died during the experiment so some values are based on only 9 rats.
Each rat was housed alone in a 25 × 18 × 19 cm hanging cage, with stainless steel back and side walls and a mesh front wall and floor. Powdered AIN-76A diet (Dyets Inc.; catalog no. 100000) was continuously available from a 4-oz glass jar (Qorpak brand) that was attached with a stainless steel spring to the front wall. Deionized water was available from a 300-mL glass bottle equipped with a neoprene stopper and a stainless steel sipper.
Procedures
Because of the large number of rats involved, the experiment was conducted in 3 replications, involving 84, 70, and 74 rats. The general design goal was for each replication to include 5 rats from the FHH strain, 5 rats from 14 or 15 consomic strains and, in the first 2 replications, 5 rats from the BN strain. Thus, the FHH strain was represented in all 3 replications, and the BN and most of the consomic strains were represented in 2 of the 3 replications. However, due to supply problems, all members of the FHH-Chr 5BN and 8BN strains were tested in a single replication, and some consomic strains had members in all 3 replications (for details, see online Supplementary material).
Starting 12–15 days after arrival, when the rats were on average 50 days old (range 42–67 days), all received a series of sixteen 2-bottle choice tests using the compounds and concentrations listed in Table 2. The concentration chosen for most compounds was the one that supported the clearest strain difference in previous work (Tordoff, Alarcon, Lawler, et al. 2008; see also online Supplementary material), with the exception that 2 concentrations of NaCl were tested (10 and 237 mM) to straddle the peak preference of the inverted U-shaped concentration-preference function of this compound (Richter 1939; Young and Falk 1956; Tordoff, Alarcon, Lawler, et al. 2008). The order of the tests (Table 2) was arranged so that, in general, compounds that were preferred were alternated with those that were avoided.
Table 2.
Taste solutions tested in parental and consomic rats, with the results of analyses of %HWI scores and estimates of the heritability of each trait
| Compound and concentration | %HWI score | h2 | r |
| One-way ANOVA | |||
| NaCl, 10 mM | F23,204 = 7.35, P < 0.0001 | 0.45 | 0.42 |
| NaCl, 237 mM | F23,203 = 5.15, P < 0.0001 | 0.37 | 0.82 |
| CaCl2, 32 mM | F23,202 = 4.59, P = 0.0009 | 0.34 | 0.93 |
| Saccharin, 1 mM | F23,204 = 6.15, P < 0.0001 | 0.27 | 0.40 |
| NH4Cl, 100 mM | F23,204 = 3.26, P < 0.0001 | 0.27 | 0.93 |
| Sucrose, 32 mM | F23,203 = 3.19, P < 0.0001 | 0.26 | 0.35 |
| KCl, 100 mM | F23,202 = 1.99, P = 0.0061 | 0.10 | 0.92 |
| Ethanol, 4% v/v | F23,203 = 4.90, P < 0.0001 | 0.36 | 0.92 |
| HCl, 1 mM | F23,203 = 1.00, NS | 0.10 | 0.87 |
| MSG, 10 mM | F23,203 = 5.43, P < 0.0001 | 0.38 | 0.43 |
| Citric acid, 1 mM | F23,200 = 1.43, P = 0.0506 | 0.15 | 0.87 |
| QHCl, 32 μM | F23,201 = 2.72, P < 0.0001 | 0.24 | 0.92 |
| Corn oil, 1% w/v | F23,201 = 2.37, P = 0.0008 | 0.21 | 0.83 |
| Denatonium, 32 μM | F23,201 = 1.08, NS | 0.11 | 0.88 |
| Polycose, 1% w/v | F23,201 = 2.01, P = 0.0057 | 0.19 | 0.42 |
| Capsaicin, 1 μM | F23,201 = 2.15, P = 0.0026 | 0.20 | 0.86 |
Compounds are listed in the order they were tested. QHCl, quinine hydrochloride; Denatonium, denatonium benzoate. Estimates of heritability (h2) in the narrow sense are based on the ratio of SSbetween strains/SStotal. r = correlation between preference scores and %HWI scores (n = 228 or slightly less). NS = not significant.
Each 2-bottle choice test was 96-h in duration. At the beginning of each test series, each rat was weighed (±0.1 g) with a top-loading balance and then returned to its cage. The rat's regular water bottle was removed and 2 similar bottles were provided, with the spouts penetrating the front wall of the cage to rest with the tips 2–4 cm above the floor and 3–4 cm apart. The bottles were initially presented so that the water was on the rat's left and taste solution on its right. The position of the 2 bottles was switched every 24 h to control for the possibility of side preferences (Bachmanov et al. 2002), and their weights (±0.1 g) were recorded at the beginning of the test, after 2 days, and at the end of the test. At the beginning and end of the test series and interspersed between each 4-day 2-bottle choice test were 24-h tests during which each rat had access to a single-weighed bottle of deionized water. This provided a measure of daily water intake and served as a washout day. With a total of 16 four-day 2-bottle tests and 17 one-day water-only tests, each replication took 81 days to complete.
We also measured the rats’ food intakes on 3 occasions: during the initial one-bottle test (when ∼7 week old), in the middle of the experiment during the one-bottle test after they received HCl (when ∼13 week old), and during the final one-bottle test (when ∼18 week old). The results of the food intake tests and body weight growth curves will be presented elsewhere (Reed DR, Duke FF, Rosazza M, Lawler MP, Alarcon LK, and Tordoff MG, manuscript in preparation).
Most solutions were made in 3-L batches by stirring the appropriate quantity of taste compound into deionized water. Ethanol solutions were made by diluting 95% (190 proof) ethanol in deionized water (i.e., vol/vol). Because of its limited solubility, capsaicin was initially dissolved in 1 mL of 95% ethanol, and this was then diluted with deionized water to produce the appropriate concentration. To avoid separation, corn oil was held in suspension with the addition of 0.3% soybean phosphatidylcholine and 0.2% xanthan gum; these were also added to the rats’ water choice, and fresh corn oil emulsions and water were provided every 24 h.
Data analyses
The change in weight of drinking bottles between measurement periods was considered a measure of fluid intake in grams. The contribution of spillage and evaporation was ignored; previous work has shown this to be <1 g/day. Intakes in grams were converted to milliliters with the assumption for all fluids that 1 g = 1 mL. Intakes from each bottle during the 96-h tests were divided by 4 to obtain average daily intakes. Intakes from both bottles were summed to obtain total fluid intakes. Values are presented as group means ± standard errors of the mean.
The percent change relative to habitual water intake score as a measure of taste solution consumption
There are technical considerations about the most appropriate metric for comparing taste solution consumption among strains of rats. Raw daily solution intakes are problematic because different strains have different fluid requirements, so solution acceptance is confounded with daily fluid intake. Body size undoubtedly plays into this relationship, and it is common to adjust intakes by dividing them by body weight. These values are provided in the online Supplementary material but we do not favor them because body weight is not a good predictor of water intake in different rat strains (see Tordoff, Alarcon, Lawler, et al. 2008 and below). A generally accepted approach to avoid the problem of differences in habitual fluid intake is to use preference scores (i.e., daily solution intakes/total daily intakes, expressed as a percentage), which have the strong advantage of being independent of daily fluid intake or other performance characteristics. However, they can be misleading due to ceiling effects and scaling problems when preferences are high (i.e., >85%). Because preference scores are ratios, they closely approximate interval scaling in the middle of the range but deviate strongly at the extremes. When preferences are high, large differences in solution intake have only small effects on preference scores, and small differences in water intake have large effects on preference scores. Another concern with using preference scores to assess taste solution consumption involves treatments or taste compounds that influence water consumption. This is a particular problem during tests involving concentrated NaCl solutions because water is consumed to dilute the osmotic load to isotonicity (Stricker 1981). To avoid these problems and to account for differences in daily fluid intakes among strains, we use here a “percent change relative to habitual water intake” (%HWI) score. This is based on solution intake during a 2-bottle test divided by intake when only water is available. The tests with only water available were the average intake during the one-bottle tests given immediately before and immediately after each 2-bottle test. We think that this %HWI score provides the most interpretable metric of an animal's response to highly preferred solutions and so present %HWI scores in the text. However, the online Supplementary material includes analyses of preference scores and provides raw intakes so that other metrics can be calculated.
Statistical analyses
For each of the 16 taste tests, the %HWI scores of the 24 strains were compared using one-way analysis of variances (ANOVAs) with strain as the factor. Strain differences were present in all cases except for the tests involving HCl and denatonium; see Table 2. The ratio of the between-strain sum of squares to the total sum of squares obtained from these analyses was used to estimate heritability (h2) in the narrow sense.
To determine whether the BN and consomic strains differed from the FHH strain, we considered any mean falling outside the 99th percentile confidence intervals of the FHH strain to be significant. We used this approach rather than post hoc tests because 1) we were interested almost exclusively in comparing each consomic strain with the FHH strain, and 2) the confidence interval can be more easily depicted graphically. The 99th percentile rather than the more usual 95th percentile interval was used to provide stronger protection against Type I errors.
Two technical issues influenced the results. First, one rat each from the FHH-Chr 6BN, 8BN, and 13BN strains died due to mechanical injury during the course of the experiment. Data from tests these rats completed are included in analyses. Second, a computer malfunction lost the one-bottle water intakes collected between the 237 mM NaCl and 32 mM CaCl2 choice tests in the second replication. To address this, the 237 mM NaCl %HWI scores and 32 mM CaCl2 %HWI scores were based on the remaining one-bottle water intakes collected either before or after these tests. (For all other tests, %HWI scores were based on the average of the one-bottle water intakes both before and after the 2-bottle choice test.)
The experiment was conducted in 3 replications (as described above). To assess the similarity of each of the replications, the one-day water intakes and %HWI scores from the 3 subgroups of 5 FHH rats were compared using one-way ANOVAs. The analyses involving MSG and citric acid %HWI scores were significant but group differences appeared to be idiosyncratic. The remaining 30 analyses did not reveal any differences, so we did not attempt to adjust measures of consumption for any systematic differences among the 3 replications.
Results
Body weight and water intake
There were marked differences among the 24 strains in body weight that persisted throughout the experiment (Table 1). The FHH strain fell near the heavy end of the strain distribution, although 2 strains were significantly heavier: the FHH-Chr 7BN strain was heavier at ∼50 days old and the FHH-Chr 5BN was heavier at both ∼50 and ∼131 days old. Most of the consomic strains had lower body weights than did the FHH group but none approached the low values of the BN strain (Table 1).
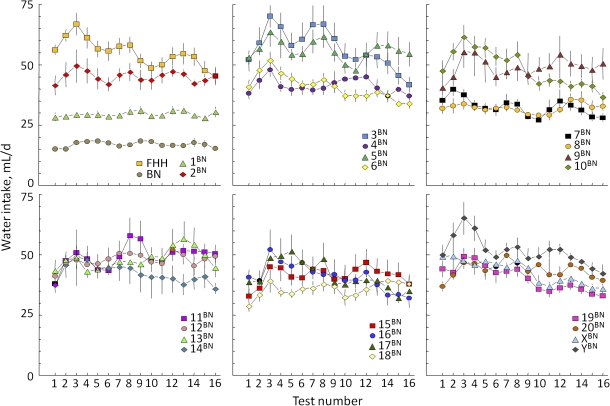
There were large and persistent differences among the strains in daily water intakes, F23,201 = 10.2, P < 0.0001; Table 1. Most strains drank progressively more water during the first 3 tests and then either maintained high intakes or gradually reduced water intakes during the subsequent 13 tests (Figure 1). However, 5 strains (BN, FHH-Chr 1BN, 8BN, 19BN, and XBN) had water intakes that did not change over the test series (Figure 1; strain × test interaction, F345,3015 = 1.52, P < 0.0001). Water intakes of all groups combined increased significantly over the first 4 tests: intakes after the 10 mM NaCl test were higher than those after the initial test, intakes after the 237 mM NaCl test were higher than those after 10 mM NaCl, and intakes after 10 mM CaCl2 were higher than those after 237 mM NaCl (main effect of test, F15,3015 = 19.4, P < 0.0001).
Figure 1.
Habitual (one-bottle) daily water intakes of FHH, BN, and FHH-Chr nBN rats. Seventeen one-bottle tests were given, starting at ∼50 days old, with one test every 5 days, interposed between 4-day 2-bottle choice tests. Vertical bars = standard errors of the mean; bars that infringe on the symbols depicting means have been removed to aid readability. This figure appears in color in the online version of Chemical Senses.
Combining water intakes on all 17 tests, the FHH strain was among a group of 9 strains with the highest water intakes (Table 1). Fourteen consomic strains had significantly lower water intakes than did the FHH strain, and the BN strain had significantly lower water intakes than did all the other groups. The correlation between average daily water intakes and average body weights was r = 0.43 (P = 0.0359; n = 24). This is consistent with the results of our survey of 14 rat strains (Tordoff, Alarcon, Lawler, et al. 2008) and, because it implies that only 18% of the variance in water intake can be accounted for by body weight, reinforces our decision not to adjust fluid intakes for body weight.
Taste solution consumption
The results obtained during 2-bottle choice tests are displayed in Figures 2–11 and summarized in Table 3. For convenience, results from different compounds are grouped according to their (putative) taste qualities, rather than in the order they were tested (as listed in Table 1). Heritability was generally low (Table 1). The results obtained with %HWI scores and preference scores were generally congruent for tests of moderately preferred or avoided taste solutions (r's = 0.82–0.93; Table 2) but differed for tests of highly preferred taste solutions (r's = 0.35–0.43; Table 2).
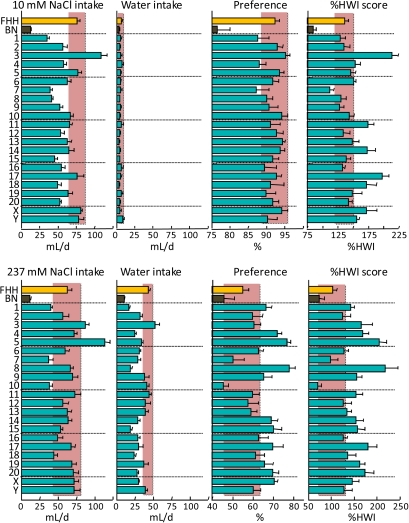
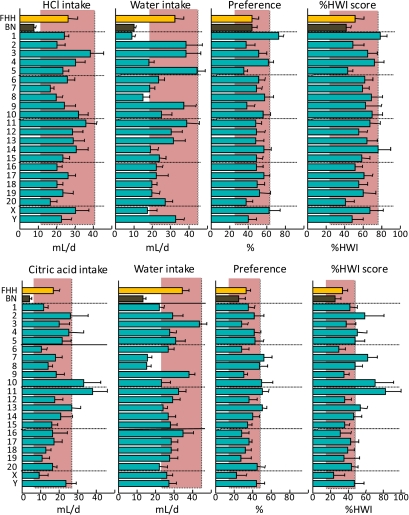
Figure 2.
Consumption of 10 mM NaCl (top) or 237 mM NaCl (bottom) and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
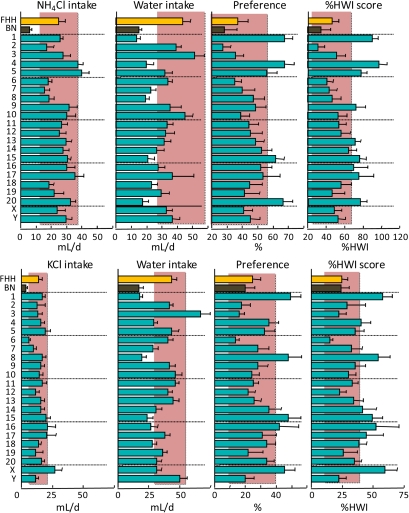
Figure 3.
Consumption of 100 mM NH4Cl (top) or 100 mM KCl (bottom) and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
Figure 4.
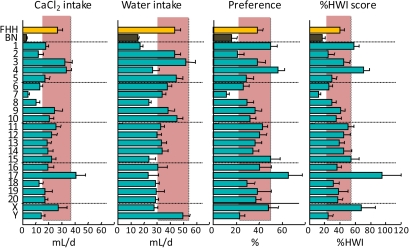
Consumption of 32 mM CaCl2 and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
Figure 5.
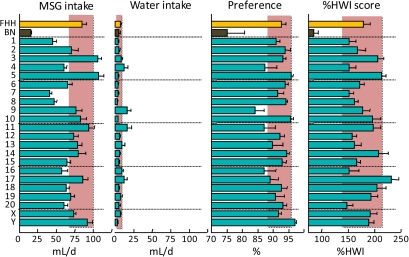
Consumption of 10 mM MSG (“umami taste”) and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
Figure 6.
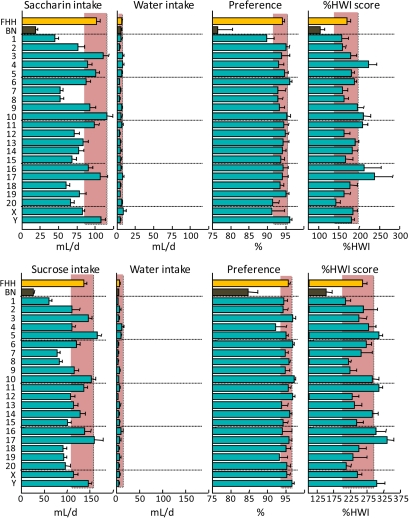
Consumption of 1 mM saccharin (top) or 32 mM sucrose (bottom) and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
Figure 7.
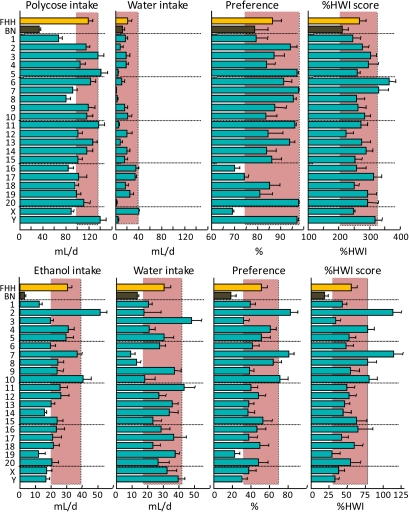
Consumption of 1% Polycose (top) and 4% ethanol (bottom) and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
Figure 8.
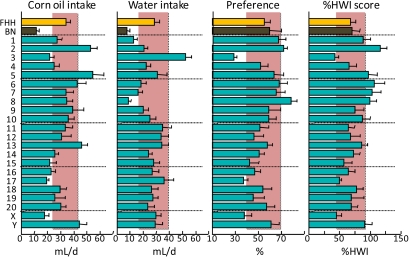
Consumption of 1% corn oil and water (with suspension agent) by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
Figure 9.
Consumption of 1 mM HCl (top) or 1 mM citric acid (bottom) and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
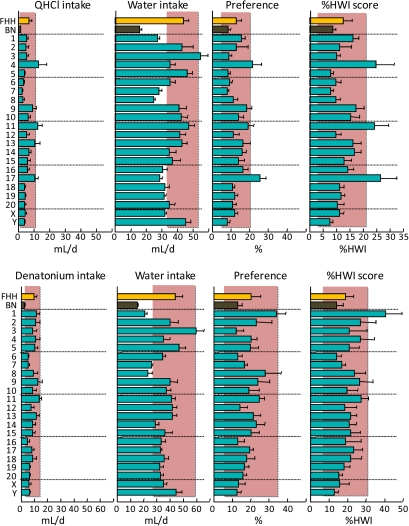
Figure 10.
Consumption of 32 μM quinine hydrochloride (QHCl; top) or 32 μM denatonium benzoate (bottom) and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
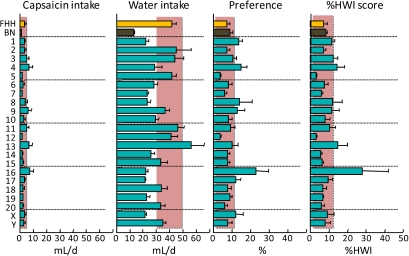
Figure 11.
Consumption of 1 μM capsaicin (a trigeminal irritant) and water by FHH, BN, and FHH-Chr nBN rats, arranged by chromosome. %HWI = Percent change relative to habitual water intake. Shaded vertical bar in each panel is 99% confidence interval of the FHH strain. This figure appears in color in the online version of Chemical Senses.
Table 3.
Summary of significant differences in %HWI scores between the FHH and other strains
| Taste | Exemplar | Strain (BN chromosome) |
||||||||||||||||||||||
| BN | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | X | Y | ||
| Salty | NaCl, 10 mM | ↓ | • | • | ↑ | ↑ | • | • | ↓ | • | • | • | ↑ | • | • | ↑ | • | • | ↑ | ↑ | • | • | ↑ | ↑ |
| NaCl, 237 mM | • | ↑ | • | ↑ | ↑ | ↑ | • | • | ↑ | ↑ | ↓ | ↑ | • | ↑ | ↑ | ↑ | • | ↑ | ↑ | ↑ | ↑ | ↑ | • | |
| Mineral | NH4Cl, 100 mM | • | ↑ | • | • | ↑ | ↑ | • | • | • | ↑ | • | • | • | ↑ | • | ↑ | ↑ | ↑ | • | • | ↑ | • | • |
| KCl, 100 mM | • | ↑ | • | • | ↑ | • | • | • | ↑ | • | • | • | • | • | ↑ | ↑ | ↑ | ↑ | • | • | • | ↑ | • | |
| CaCl2, 32 mM | ↓ | ↑ | • | • | ↑ | • | • | ↓ | • | • | • | • | • | • | • | • | • | ↑ | • | • | • | ↑ | • | |
| Umami | MSG, 10 mM | ↓ | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ↑ | • | • | • | • | • |
| Sweet | Saccharin, 1 mM | ↓ | • | • | • | ↑ | • | • | • | • | • | ↑ | ↑ | • | • | • | • | ↑ | ↑ | • | • | • | • | • |
| Sucrose, 32 mM | ↓ | • | • | • | • | ↑ | • | • | • | • | • | ↑ | • | • | • | • | ↑ | ↑ | • | • | • | • | ↑ | |
| Carbohydrate | Polycose, 1% | • | • | • | • | • | • | ↑ | ↑ | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Mixed | Ethanol, 4% | ↓ | • | ↑ | • | • | • | • | ↑ | • | • | ↑ | • | • | • | • | • | • | • | • | ↓ | • | • | • |
| Fat | Corn oil, 1% | • | • | ↑ | ↓ | • | ↑ | ↑ | ↑ | ↑ | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Sour | HCl, 1 mM | • | ↑ | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| Citric acid, 1 mM | • | • | ↑ | • | ↑ | • | • | ↑ | • | • | ↑ | ↑ | • | ↑ | • | • | • | • | • | • | • | • | • | |
| Bitter | QHCl, 32 μM | • | • | • | • | ↑ | • | • | • | • | • | • | ↑ | • | • | • | • | • | ↑ | • | • | • | • | • |
| Denatonium, 32 μM | • | ↑ | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |
| Trigeminal | Capsaicin, 1 μM | • | • | • | • | ↑ | • | • | • | • | • | • | • | • | ↑ | • | • | ↑ | • | • | • | • | • | • |
↓ = P < 0.01, lower than FHH strain, ↑ = P < 0.01, higher than FHH strain, • = not different from FHH strain. A significant difference implies that the chromosome involved harbors at least one QTL influencing the phenotype.
Discussion
We have identified some of the chromosomes that harbor genes responsible for the variation in taste solution consumption among FHH-Chr nBN rats. In the sense that an identified chromosome is a quantitative trait locus (QTL), we discovered a total of 84 QTLs, with between 1 and 16 QTLs influencing each of the 16 taste phenotypes examined. The use of consomic rats is relatively new, so below we first discuss methodological considerations of the approach and address some of the limitations inherent with the phenotypes measured here. We then discuss the implications of our findings for the acceptance of water and each taste solution, particularly in the context of what is already known about taste transduction mechanisms. We end with a more general discussion of the implications of the results for the genetic architecture of taste solution consumption.
Methodological considerations
Interpretation of data from consomic rats
Consomic rodent strain sets are relatively new and underutilized tools for the dissection of genetic traits (for reviews, see Nadeau et al. 2000; Cowley, Liang, et al. 2004; Cowley, Roman, and Jacob 2004; Hill et al. 2006; Gregorova et al. 2008). Several consomic rodent strains have been developed but only 5 complete sets are extant, 2 of rats (FHH-Chr nBN and SS-Chr nBN [PhysGen 2009]) and 3 of mice (C57BL/6J-Chr nA/J [Singer et al. 2004], C57BL/6J-Chr nPWD/ForeJ [Gregorova et al. 2008], and DBA/2-Chr nDU6i [Bevova et al. 2006]). To our knowledge, ours is the first study to screen a complete set of consomic rats with behavioral traits and the first in either species to examine taste preferences. Most previous work on the genetic basis of taste preferences has involved testing segregating hybrid mice followed by the generation of congenic lines to isolate QTLs (e.g., Tordoff 2008). The use of consomic strains has several advantages over this older approach. First, experimental designs using segregating generations require comparisons to be made between individuals, whereas designs using consomic animals allow comparisons between strains. The use of groups rather than individuals yields greater statistical power; this is particularly important for behavioral phenotypes because these tend to be more susceptible to environmental and experiential influences than are physiological or anatomical phenotypes and thus more variable. Second, each consomic strain has a defined genetic background consisting of a mosaic of its 2 parental strains. Recombinant inbred strains also have this property but a consomic strain set has 2 additional advantages: 1) the genotype of each parental strain is represented over the entire genome in a systematic manner and 2) the results obtained are easier to analyze and, arguably, simpler to conceptualize: Differences between a consomic strain and its parental background strain imply that a gene or genes on the introgressed chromosome influences the trait under study. Third, to isolate QTLs, it is easier and faster to develop congenic lines from consomic strains than from segregating hybrids: Typically, the process requires 10 generations if starting from segregating hybrids but only 2 if starting from consomic strains. There is also less chance of “losing” a phenotype due to the loss of epistatic or background effects while breeding congenics from consomics because the locus of interest is already expressed on a background that is identical in all but the introgressed chromosome.
Limitations of the approach
The consomic approach relies on the implication that differences in a particular trait between a consomic strain and its parental background strain are the result of a gene or genes on the introgressed chromosome. However, the number of loci on the introgressed chromosome is unknown. Moreover, the lack of a difference between a consomic strain and its parental background strain is ambiguous: The failure to detect a difference could be due to 1) the absence of functional polymorphisms on the introgressed chromosome, 2) the inevitable lack of statistical power associated with the difficulty of proving the null hypothesis, or 3) genes with opposing or antagonistic effects located on the introgressed chromosome. Consomic strains also do not preserve QTLs involving polychromosomal interactions (e.g., genes requiring transregulatory elements or epistatic genes on different chromosomes). Consequently, the consomic approach can be used to identify the chromosomal locations of some, but not all, genes influencing a trait.
Two-bottle choice test phenotype
The phenotypes examined in this study are based on the long-term 2-bottle choice test. This is straightforward and inexpensive to conduct, and preferences measured using it reflect the food choices of animals foraging in the wild (for reviews, see Jacobs et al. 1978; Shumake 1978; Provenza 1996; Stephens et al. 2007). Taste is a dominant contributor to taste solution consumption under most circumstances, particularly when the animals are fed a nutritionally complete diet, as they were here. However, fluid intakes in long-term 2-bottle choice tests are also influenced by postingestive, experiential, cognitive, and nongustatory orosensory cues. Very little is known about the genetic architecture of these nontaste influences on taste solution consumption, with the possible exception of those related to ethanol consumption (see Ethanol, below). In contrast, there have been several recent discoveries of taste transduction and processing mechanisms in the mouse. Consequently, in the following discussion, we focus on comparing our results with these new findings.
This article introduces the %HWI score—solution intake relative to habitual water intake—as a metric of the avidity of rats for taste solutions. For tests involving taste solutions that are ingested in moderate volumes, %HWI scores closely reflected the more familiar preference scores (r's = 0.82–0.93; Table 2). For these taste compounds, when a consomic strain differed from the FHH strain in %HWI score it usually also differed in preference score; the exceptions were when the strain mean fell close to the confidence interval of the FHH strain so that one score was marginally significant and the other marginally nonsignificant. However, for tests of highly preferred taste solutions, the correspondence between %HWI scores and preference scores was weak (r's = 0.35–0.43; Table 2), and there were several examples where a consomic strain had clearly significant %HWI scores but no obvious trend in preference scores. For strongly preferred solutions, there was greater constraint on the range of preference scores than %HWI scores. For example, the 10 mM NaCl preference scores of the FHH and consomic strains fell between 87% and 95%, and it is doubtful that differences between strains within this range have any meaningful (as opposed to statistical) significance. In contrast, the equivalent %HWI scores fell between 84 and 197 %HWI, exposing large strain differences. We see few disadvantages of the %HWI score. One is that it requires making measurements of daily water intake, which is time consuming. Another is that water intakes measured on days soon after a taste solution is consumed are open to contamination by carryover effects. This is a potential source of error here because the average of pre- and post-choice test water intakes was used to calculate %HWI scores. However, daily water intakes were fairly stable from test-to-test (Figure 1), which implies that if any carryover effects were present then they had minimal influence on the results.
Chromosomes responsible for the consumption of water and individual taste compounds
Habitual (daily) water intake
We measured the parental and consomic rats’ daily water intakes on 17 occasions, from ∼7 to 18 week of age. Most strains progressively increased daily water intakes up to ∼9 week of age then progressively decreased them over the following 9 week. The FHH strain is known to develop hypertension, with its onset coincident with increased daily water intakes (Kuijpers et al. 1986). We did not measure blood pressure in our study but it would not be surprising to find that the onset of hypertension occurred at about 9 week of age, raising the possibility of pleiotropy. However, the FHH-Chr 1BN, 8BN, 19BN, and XBN strains did not increase water intake but are susceptible to hypertension (Mattson et al. 2007), and the FHH-Chr 20BN strain increased water intake but is resistant to hypertension (Mattson et al. 2007). Thus, water intake and hypertension can be genetically dissociated.
The FHH strain is also susceptible to kidney disease (Mattson et al. 2007). Consequently, a “leaky kidney” could feasibly account for the FHH strain’s high water intake and perhaps even its avidity for some taste solutions. Mattson et al. (2007) found that the FHH-Chr 1BN, 14BN, 15BN, 16BN, 18BN, and 20BN strains had reduced kidney disease relative to the FHH strain. In the present study, all 6 of these consomic strains had reduced water intakes relative to the FHH strain, which is consistent with the leaky kidney explanation. However, some strains (e.g., FHH-Chr 7BN and 8BN) are apparently not protected from kidney disease but had low water intakes. Thus, there was not a simple relationship between kidney disease and water intake. It is likely that different mechanisms underlie kidney disease on different chromosomes so, for example, the locus on Chr 1 that influences water intake may do so by causing kidney leakage, but loci on other chromosomes may involve other mechanisms. Kidney disease may be one cause of high water intakes but it is not the only one.
Sodium-selective saltiness (NaCl)
There are at least 2 types of sodium-sensitive taste transduction mechanisms in rodents: one is highly selective for sodium and lithium salts and is mediated by sodium-selective “N”-fibers (e.g., Heck et al. 1984; Brand et al. 1985; DeSimone and Ferrell 1985; Ninomiya et al. 1989; Hettinger and Frank 1990; Roitman and Bernstein 1999; Chandrashekar et al. 2010) and the other is nonselective among sodium, potassium, and ammonium salts and is mediated by generalist cation-sensitive nerve fibers, sometimes called H- or E-fibers (e.g., Formaker and Hill 1988; Roitman and Bernstein 1999; Lyall et al. 2004; see General salt taste, below). The sodium-selective transduction mechanism involves epithelial sodium channels (ENaCs) that can be debilitated by amiloride (e.g., Chandrashekar et al. 2010). In mouse taste tissue, there are 3 ENaC subunits, Scnn1a, Scnn1b, and Scnn1g. In rats, Sccn1a is located on Chr 4 and both Scnn1b and Scnn1g are located on Chr 1. The consomic strains involving these chromosomes had NaCl consumption scores different from those of the FHH strain, which is consistent with a contribution of ENaCs to sodium taste. However, the same chromosomes also influenced consumption of NH4Cl, KCl, and CaCl2, which do not influence ENaCs (Brand et al. 1985; DeSimone and Ferrell 1985). Thus, the most parsimonious explanation is that generalist cation-sensitive mechanisms are responsible for the QTLs on these chromosomes, although we cannot rule out the possibility that sodium-specific mechanisms contribute as well.
It is noteworthy that several mouse strains voluntarily consume NaCl but do not have amiloride-sensitive sodium taste, and the evidence that human sodium taste is amiloride sensitive is equivocal (e.g., Ossebaard and Smith 1996; Anand and Zuniga 1997; Halpern 1998) so the existence of additional sodium-specific taste transduction mechanisms seems likely. One possibility involves Mcoln3 (aka TRPML3 and SNMX-34), which has been described as a human sodium taste receptor (Moyer et al. 2009). However, this gene is located on rat Chr 2, a chromosome that did not influence NaCl consumption in the present study.
As noted above, the FHH and some consomic strains were probably hypertensive (e.g., Kuijpers et al. 1986; Mattson et al. 2007). Hypertension has been linked to NaCl preference, based primarily on the observation that the SHR strain is both hypertensive and an avid consumer of NaCl (e.g., Catalanotto et al. 1972). This is also true of the FHH strain, but it is probably due to coincident fixation of the traits during inbreeding. In male rats, only substitution of Chr 20 significantly reduced mean arterial pressure (Mattson et al. 2007) but in our work these rats had FHH-like 10 mM NaCl consumption scores and exacerbated 237 mM NaCl consumption scores. Thus, as is the case with the SHR strain (Di Nicolantonio et al. 1983; Yongue and Myers 1989), blood pressure and NaCl preference can be genetically dissociated.
Most rat strains display an inverted U-shaped concentration-preference function for NaCl, with the peak preference being ∼150 mM (Richter 1939; Young and Falk 1956; Tordoff, Alarcon, Lawler, et al. 2008). The BN and FHH strains are typical in this respect (Tordoff, Alarcon, Lawler, et al. 2008) and the avidity for NaCl is higher in the FHH than in the BN strain. We found here that several consomic strains have even greater avidity for NaCl than does the FHH strain. Chr 3, 4, 11, 14, 17, 18, and X were implicated in the response to both NaCl concentrations tested, Chr 7 and Y were implicated in the response to 10 mM NaCl only, and Chr 1, 5, 8, 9, 10, 13, 15, 19, and 20 were implicated in the response to 237 mM NaCl only. The involvement of different loci in the response to different concentrations of NaCl is to be expected because at least some of the mechanisms controlling NaCl consumption are likely to be concentration-specific. Given that 10 mM NaCl is hypotonic and 237 mM NaCl is hypertonic, one interpretation is that the loci implicated in the response to 10 mM NaCl mediate taste-related mechanisms, whereas those implicated specifically in the response to 237 mM NaCl mediate mechanisms related to the osmotic or other postingestive effects of NaCl. The finding that 18 of the 22 consomic strains—all except those involving Chr 2, 6, 12, and 16—influenced the ingestion of one or both concentrations of NaCl implies that at least 18 genes are involved in this phenotype, but even this is likely an underestimate of the complexity of the genetic architecture involved because some chromosomes may harbor more than one pertinent gene. Clearly, the controls of NaCl consumption are dauntingly complex, but perhaps this is to be expected given the many crucial roles that sodium plays in homeostasis.
General salt taste (NH4Cl and KCl)
The mechanisms underlying the transduction of nonsodium salts are unclear. The saltiness of these compounds has been attributed to amiloride-insensitive generalist salt taste mechanisms, and it may involve paracellular pathways (Elliott and Simon 1990) and/or TRPV1 (aka VR-1) receptors (Lyall, Heck, Phan, et al. 2005; Lyall, Heck, Vinnikova, et al. 2005). However, Trpv1, the gene encoding for TRPV1, did not appear to influence consumption of NH4Cl or KCl in the present study: Trpv1 is located on rat Chr 10, but the FHH-Chr 10BN strain had FHH-like intakes of NH4Cl and KCl. Instead, loci on Chr 1, 4, 15, 16, and 17 influenced %HWI scores for both NH4Cl and KCl, loci on Chr 5, 9, 13, and 20 influenced %HWI scores for NH4Cl alone, and loci on Chr 8, 14, and X influenced %HWI scores for KCl alone. In all cases, the consomic strains had higher %HWI scores than did the FHH strain.
The involvement of a shared amiloride-insensitive salt transduction mechanism would be reflected in a common response to NH4Cl, KCl, and NaCl. The FHH-Chr 1BN, 4BN, 15BN, and 17BN strains fulfill this because each had elevated %HWI scores to all 3 monovalent chlorides. In addition to saltiness, a bitter component of KCl is recognized, and Chr 1, 4, and 17 were implicated in the response to denatonium or quinine, raising the possibility of pleiotropy. KCl is known to influence voltage-gated calcium channels in taste buds (Hacker et al. 2008). There are ∼15 subunits of these channels, with genes located on rat Chr 3, 4, 10, 13, 16, and 19, so there is the possibility of involvement of some of these (i.e., Cacna1c, Cacna2d1, and CaCna2d4 on Chr 4 and Cacna1e on Chr 13). There is also evidence for a potassium appetite in rats that is distinct from sodium appetite, but the specificity and detection mechanisms involved are unclear (e.g., Milner and Zucker 1965; Guenthner et al. 2008). A likely location of genes responsible for an action specific to KCl is on Chr 16 because only the FHH-Chr 16BN strain differed from the FHH strain in KCl %HWI scores but not NaCl %HWI scores.
Calcium (CaCl2)
The taste of calcium is distinct from that of other salts (e.g., Coldwell and Tordoff 1996; McCaughey and Tordoff 2002; McCaughey et al. 2005). A genetic analysis based on C57BL/6J and PWK/PhJ F2 mice identified 2 calcium taste receptor genes (Tordoff et al. 2007a; Tordoff 2008; Tordoff, Reed, and Shao 2008; Tordoff, Shao, et al. 2008): Tas1r3, which has also been implicated in sweet and umami taste (see Umami and Sweet, below) and Casr, which is also key to the regulation of blood calcium concentrations. Both receptors are found in taste buds (Nelson et al. 2001; San Gabriel et al. 2009), and it has recently been suggested that CaSR mediates kokumi taste (Ohsu et al. 2009), an orosensory quality recognized in Japan but unknown in the West. We found here that the FHH strain had substantially higher avidity for CaCl2 than did the FHH strain but this was unlikely to involve either Tas1r3 or Casr: Tas1r3 is located on Chr 5 and Casr on Chr 11 in the rat, and the corresponding consomic strains had FHH-like CaCl2 %HWI scores. Instead, the phenotypic difference was captured completely by the FHH-Chr 7BN strain. There are, of course, many genes on Chr 7 that could potentially influence calcium consumption but a particularly attractive candidate is Vdr, the 1,25-dihydroxyvitamin D3 receptor gene, which is a key controller of calcium metabolism, and its human ortholog has many functional mutations. In contrast to the locus on Chr 7, loci on Chr 1, 4, 17, and X increased %HWI scores; the identities of these QTLs are unknown.
Umami (MSG)
Umami, the “fifth” basic taste, is most often exemplified by the taste of MSG. In mice, umami transduction is mediated by at least 2 distinct receptor types. One is a G-protein–coupled receptor dimer involving T1R1 and T1R3 (Zhao et al. 2003; Chandrashekar et al. 2006), with genes Tas1r1 and Tas1r3, located on rat Chr 5. The other involves metabotropic glutamate receptors (most likely truncated forms of Grm1, aka mGluR1, or Grm4, aka mGluR4 [Delay et al. 2009]), with genes located on rat Chr 1 and 20. We found considerable variation in the response to MSG, with FHH rats consuming more than twice as much MSG as did BN rats, and strain means ranging from 86 ± 9 %HWI (BN strain) to 231 ± 14 %HWI (FHH-Chr 17BN strain). However, it is unlikely that genes on Chr 1, 5, or 20 were involved because only the FHH-Chr 17BN strain differed significantly from the FHH strain.
Sweet (saccharin and sucrose)
In the mouse, the transduction of sweet taste is mediated primarily by a G-protein–coupled receptor dimer involving T1R2 and T1R3. These receptor subunits are encoded by the genes Tas1r2 and Tas1r3, which are located on rat Chr 5. Polymorphisms in the sequence of Tas1r3 account for most of the variation in sweet solution intake among inbred mouse strains (Bachmanov, Li, et al. 2001; Reed et al. 2004). However, other mechanisms must also be involved because the preference for high concentrations of some sweeteners persists in Tas1r3 null mice (Damak et al. 2003). Moreover, there is no relationship between sequence variation of Tas1r3 and sweet preference in rats (Lu et al. 2005). Consistent with this, we found that the BN strain had substantially lower avidity for both saccharin and sucrose than did the FHH strain but that the FHH-Chr 5BN strain had FHH-like saccharin %HWI scores and elevated sucrose %HWI scores. Thus, the difference in response between the parental strains cannot easily be ascribed to either Tas1r2 or Tas1r3.
Instead, variation in response to sweeteners was due to QTLs on Chr 4, 5, 10, 11, 16, 17, and Y. Of these, the loci on Chr 11, 16, and 17 influenced both saccharin and sucrose consumption. Given the structural, chemical, osmotic, and energetic differences between the 2 sweeteners, these loci appear to be the strongest candidates to be involved in the response to sweetness per se. The loci on Chr 5 and Y were specific to sucrose consumption, whereas those on Chr 4 and 10 were specific to saccharin consumption. Perhaps the locus on Chr 4 is involved in the response to saccharin's bitter aftertaste (Dess 1993) because this chromosome also influenced QHCl consumption.
G-Protein–coupled receptor intracellular signaling cascade
G-protein–coupled receptors responsible for the transduction of sweet, umami, calcium, and bitter taste initiate an intracellular signaling cascade involving gustducin, TRPM5, and Plcb2 (Hacker et al. 2008). Gustducin is encoded by Gnat3, which resides on rat Chr 4. The FHH-Chr 4BN strain drank more saccharin, CaCl2, and QHCl than did the FHH strain, but we suspect this is coincidental rather than due to the participation of Gnat3: the FHH-Chr 4BN strain also drank more NaCl, NH4Cl, KCl, and capsaicin, which do not involve G-protein–coupled receptors, and it did not drink more sucrose, MSG, or denatonium, which do. Trpm5 is located on rat Chr 1, and Plcb2 is located on rat Chr 3, and neither of these chromosomes influenced the response to saccharin, sucrose, MSG, calcium, or QHCl. Thus, it appears unlikely that functional polymorphisms in Gnat3, Trpm5, or Plcb2 are responsible for the variation in the rats’ responses to sweet, umami, calcium, or bitter tastes.
Polycose
Polycose is a soluble mixture of polysaccharides derived from corn starch. Consistent with results from other strains of rats (e.g., Tordoff, Alarcon, Lawler, et al. 2008), the FHH and BN rat strains were avid consumers of Polycose. The receptors responsible for guiding Polycose consumption are unknown, although strong evidence indicates that they are distinct from those used to detect other carbohydrates, including simple sugars and starches (for reviews, see Sclafani 1987, 2004). Our results implicate genes on Chr 6 and 7 in the response to Polycose. Neither of these chromosomes was implicated in the response to saccharin or sucrose, which further attests to the existence of distinct controls for the consumption of Polycose and sweeteners.
Ethanol
In contrast to the other taste compounds studied here, there is wide-ranging work on the genetic controls of ethanol consumption. This has implicated genes related to γ-aminobutyric acid, dopamine, corticotropin-releasing factor, opioids, cannabinoids, serotonin, various ion channels, adenosine, cyclase-related genes, protein kinases, glutamate, various neuropeptides and chemokines, as well as others (for a review of 93 alcohol-related genes, see Crabbe et al. 2006). Chemosensory-related genes implicated in ethanol consumption by mice include Tas1r3, Trpv1, Gnat3, and Trpm5 (Blednov et al. 2008; Blednov and Harris 2009), which may all be involved in detecting ethanol's sweet component. Undoubtedly, there are more.
Consumption of 4% ethanol ranged widely among the 24 strains tested, from strong avoidance by BN rats (18 ± 5 %HWI), through indifference by the FHH strain (56 ± 8 %HWI), to strong acceptance by the FHH-Chr 2BN strain (114 ± 11 %HWI). Relative to the FHH strain, the FHH-Chr 19BN strain had lower avidity for ethanol, whereas the FHH-Chr 2BN, 7BN, and 10BN strains had higher avidity for ethanol. There are no obvious taste-related genes but there are many candidate genes with actions in the brain that could be responsible for these strain differences (Crabbe et al. 2006).
Fat (corn oil)
The existence of orosensory fat detectors is controversial but becoming increasingly accepted, primarily due to the discovery of several putative transduction mechanisms (for reviews, see Laugerette et al. 2007; Mizushige et al. 2007; Gaillard, Passilly-Degrace, and Besnard 2008; Khan and Besnard 2009; Mattes 2009). Early work implicated delayed rectifying potassium channels (Gilbertson et al. 1997), although the specific type(s) involved is unclear. More attention has been paid to the cell-surface membrane protein, CD36 (e.g., Laugerette et al. 2005; Gaillard, Laugerette, et al. 2008; Khan and Besnard 2009), which is encoded by the Cd36 gene on rat Chr 4. Less strong data link fat detection to Ffar1 (aka GPR40) and Gpr120 [(Matsumura et al. 2007); both on rat Chr 1]. The results obtained here implicate genes on Chr 2, 3, 5, 6, 7, and 8 but not the chromosomes harboring Cd36, Ffar1, or Gpr120. Clearly then, there are mechanisms influencing fat consumption that remain to be discovered. Texture and odor make important contributions to the recognition of fat by rats (e.g., Ramirez 1992, 1993, 1994) so it would not be surprising to find that some of the linkages obtained here are involved with these sensations.
Sour (HCl and citric acid)
Sour (acid) taste in the mouse is thought to be mediated by the receptor potential channel, PKD2L1, perhaps only when coexpressed with PKD1L3 (Ishimaru et al. 2006). However, neither gene encoding these channels appears to influence the phenotype observed here: In the rat, Pkd2l1 is located on Chr 1, and this chromosome influenced the response to HCl but not citric acid. Pkd1l3 is located on Chr 19, and this chromosome did not influence the response to either acid tested. Several other genes have been implicated in sour taste but these can also be excluded by virtue of their location on chromosomes that did not cause an abnormal phenotype, including Kcnk3 (aka TASK-1) on Chr 6, Slc9a1 (aka NHE-1; the Na+/H+ amiloride-sensitive solute carrier) on Chr 5, Hcn1 (the hyperpolarization-activated cyclic nucleotide-gated potassium channel 1) on Chr 3, Hcn4 on Chr 8, and Glp1r (glucagon-like peptide 1 receptor) on Chr 20 (for a review, see Bachmanov and Beauchamp 2007). The candidate sour taste receptor gene Accn1 (aka ASIC2a/ASIC2b; Shimada et al. 2006) is located on Chr 10, coincident with a consomic strain displaying high citric acid %HWI scores but this did not extend to HCl %HWI scores. Indeed, only the FHH-Chr 1BN strain differed from the FHH strain in HCl %HWI scores, and the effect size here was small (the omnibus ANOVA was not significant). This reduces enthusiasm for using the FHH-Chr nBN strains to isolate genes responsible for sour taste.
Bitter (QHCl and denatonium)
Bitter taste is mediated by a family of ∼30 T2R receptors, with most having genes located on Chr 2 or 4 in the rat. Consistent with a contribution of one or more of the T2R receptors in the present experiment, the FHH-Chr 4BN strain had QHCl %HWI scores that were significantly higher than those of the FHH strain. There were additional effects on QHCl %HWI scores involving Chr 11 and 17 and denatonium %HWI scores involving Chr 1. There are no obvious candidate genes for these loci. The lack of coincidence between QTLs involving QHCl and denatonium is consistent with the involvement of different receptors (Meyerhof et al. 2010); however, behavioral data from rats and humans suggest that the 2 have similar bitter tastes (Delwiche et al. 2001; Brasser et al. 2005).
Trigeminal (capsaicin)
In mice, the “burning” or “spicy” sensation produced by oral capsaicin is believed to be mediated by TRPV1 receptors in trigeminal nerve endings (e.g., Liu and Simon 1996). However, the TRPV1 receptor does not appear to influence the variation in capsaicin consumption observed here; the Trpv1 gene is located on Chr 10 but the FHH-Chr 10BN strain had FHH-like capsaicin consumption. Instead, Chr 4, 13, and 16 were implicated. The genes underlying the QTLs on these chromosomes are unknown.
General discussion
Relative to the FHH strain, the BN strain had lower avidity for most of the taste solutions tested in this experiment. It is therefore possible that the BN strain has a gene variant that causes a general avoidance of taste solutions. Possibilities could include a gene responsible for heightened neophobia (avoidance of novelty) or heightened preference for water. Although no chromosome was implicated in the response to all taste solutions, the FHH-Chr 4BN and 17BN strains each had elevated %HWI scores for the majority of taste solutions tested. These results cluster considerably more than would be expected if randomly located genes influenced each trait independently, so we suspect that there are pleiotropic influences at play on Chr 4 and 17, but it is unclear why these affect consumption of some taste solutions but not others (see Table 3).
The results from the BN and FHH parental strains imply that, overall, genes from the BN strain tend to reduce taste solution consumption. It is therefore counterintuitive that the direction of 79 of the 84 QTLs discovered involved consomic strains having higher %HWI scores than did the FHH strain. One might expect a bias in this direction if all the taste compounds tested were avoided by the FHH strain but this was not the case: for the 6 compounds preferred more than water (i.e., 10 and 237 mM NaCl, MSG, saccharin, sucrose, and Polycose), the consomics had higher %HWI scores than did the FHH strain in 36 of 38 significant differences; for the 10 compounds preferred less than water, the consomics had higher %HWI scores than did the FHH strain in 43 of 46 significant differences. Thus, the general effect of introgressed BN chromosomes was to increase acceptance of tastes, not to cause a more extreme phenotype, as might be expected if the FHH strain had a widespread loss of the ability to detect tastes. Notably, the sum of the strain effects expressed in individual consomic strains was far greater than the difference between the parental strains. This phenomenon, which has been observed for more than 60 polygenic traits (Mattson et al. 2007; Shao et al. 2008), is crucial for gene discovery because it implies that a gene making a minor or latent contribution to a trait in the parental strains can be studied in the appropriate consomic strain.
This experiment uncovered many QTLs but even this long list must inevitably be incomplete. First, the QTLs were determined by a moderately stringent statistical criterion; a looser criterion would have introduced many additional ones. Second, the number of rats tested was relatively small. With more animals and thus more statistical power, more QTLs would be discovered, albeit with each contributing smaller effects than the ones already found. Third, at least some of the chromosomal effects will ultimately resolve into 2 or more independent QTLs on the same chromosome. Fourth, some QTLs will have been missed because a QTL with the opposite effect was located on the same chromosome or because trans-chromosomal epistasis was disrupted. Fifth, the phenotype was based on only one concentration of each taste solution (2 for NaCl), so genes exerting effects specifically at lower or higher concentrations would be missed. Sixth, genetic variation was limited to polymorphisms between the FHH and BN parental strains. Consequently, genes that do not involve functional polymorphisms between these 2 strains would not be detected even though they may be important determinants of phenotypic variation among other strains or other species. We conclude that the present work provides a list of chromosomal locations to begin the search for genes responsible for taste solution consumption but the list is far from complete.
Given the large number of chromosomes implicated, we were surprised by how rarely genes known to be involved in taste perception in the mouse resided on chromosomes contributing to the variation in taste solution acceptance of the consomic strains. In particular, we found little-or-no evidence for a contribution of Scnn1a, Scnn1b, or Scnn1g to NaCl acceptance, Casr or Tas1r3 to calcium acceptance, Tas1r1, Tas1r2, Tas1r3, Gnat3, Trpm5, or Plcb2 to sweet or umami acceptance, Cd36, Ffar1, or Gpr120 to fat acceptance, Pkd2l1 or Pkd1l3 to sour acceptance, or Trpv1 to capsaicin acceptance. As discussed above, our conclusions are made from the absence of an effect of an introgressed chromosome on a trait, so it is possible that some of the genes listed above are involved but their effects are subtle or masked by genes on the same chromosome with antagonistic effects. However, it seems very unlikely that this explains all the “discrepancies.” Instead, we suspect that the mechanisms responsible for taste preference variation among the FHH-Chr nBN rat strains differ substantially from those responsible for taste preference variation in the mouse. The limited generalization from mouse to rat raises questions about how well results found in either rodent species will generalize to humans. At the very least, the current results demonstrate that the genetic architecture of taste preferences is likely to be considerably more complex than has heretofore been acknowledged.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health [grant number DK-46791]; and by the National Institute on Deafness and Other Communication Disorders at the National Institutes of Health [grant number DC-10149]. The consomic rats used in this study were provided by a Seed Grant Program sponsored by Physiogenix Inc.
Supplementary Material
Acknowledgments
Skillful technical assistance was provided by Laura K. Alarcón, Laura H. Cox, Yaritza Davila, Hillary T. Ellis, Adam Faranda, Dionne Heard, Maureen P. Lawler, Vishaal Patel, Matthew R. Rosazza, and Mary M. Shang. Drs Danielle Reed and Alexander Bachmanov provided useful comments that improved earlier drafts of this paper.
References
- Anand KK, Zuniga JR. Effect of amiloride on suprathreshold NaCl, LiCl, and KCl salt taste in humans. Physiol Behav. 1997;62:925–929. doi: 10.1016/s0031-9384(97)00174-1. [DOI] [PubMed] [Google Scholar]
- Aulakh CS, Tolliver T, Wozniak KM, Hill JL, Murphy DL. Functional and biochemical evidence for altered serotonergic function in the fawn-hooded rat strain. Pharmacol Biochem Behav. 1994;49:615–620. doi: 10.1016/0091-3057(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK, Tordoff MG. Voluntary consumption of NaCl, KCl, CaCl2 and NH4Cl solutions by 28 mouse strains. Behav Genet. 2002;32:445–457. doi: 10.1023/a:1020832327983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Hajnal A. Male rats show an indifference-avoidance response for increasing concentrations of the artificial sweetener sucralose. Nutr Res. 2005;25:693–699. doi: 10.1016/j.nutres.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevova MR, Aulchenko YS, Aksu S, Renne U, Brockmann GA. Chromosome-wise dissection of the genome of the extremely big mouse line DU6i. Genetics. 2006;172:401–410. doi: 10.1534/genetics.104.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacol. 2009;56:814–820. doi: 10.1016/j.neuropharm.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter JD, Jr, Bachmanov AA. Genetics and evolution of taste. In: Firestein S, Beauchamp GK, editors. The senses: a comprehensive reference, volume 4, olfaction and taste. San Diego: Academic Press; 2008. 371–390. [Google Scholar]
- Brand JG, Teeter JH, Silver WL. Inhibition by amiloride of chorda tympani responses evoked by monovalent salts. Brain Res. 1985;334:207–214. doi: 10.1016/0006-8993(85)90212-4. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Mozhui K, Smith DV. Differential covariation in taste responsiveness to bitter stimuli in rats. Chem Senses. 2005;30:793–799. doi: 10.1093/chemse/bji071. [DOI] [PubMed] [Google Scholar]
- Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ. Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet. 1996;12:44–51. doi: 10.1038/ng0196-44. [DOI] [PubMed] [Google Scholar]
- Catalanotto F, Schecter PJ, Henkin RI. Preference for NaCl in the spontaneously hypertensive rat. Life Sci. 1972;11:557–564. doi: 10.1016/0024-3205(72)90190-7. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldwell SE, Tordoff MG. Immediate acceptance of minerals and HCl by calcium-deprived rats: brief exposure tests. Am J Physiol Regul Integr Comp Physiol. 1996;271:R11–R17. doi: 10.1152/ajpregu.1996.271.1.R11. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand. 2004;181:585–592. doi: 10.1111/j.1365-201X.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Roman RJ, Jacob HJ. Application of chromosomal substitution techniques in gene-function discovery. J Physiol. 2004;554:46–55. doi: 10.1113/jphysiol.2003.052613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Daoust M, Compagnon P, Legrand E, Boucly P. Ethanol intake and 3H-serotonin uptake I: a study in fawn-hooded rats. Life Sci. 1991;48:1969–1976. doi: 10.1016/0024-3205(91)90230-9. [DOI] [PubMed] [Google Scholar]
- Datta YH, Wu FC, Dumas PC, Rangel-Filho A, Datta MW, Ning G, Cooley BC, Majewski RR, Provoost AP, Jacob HJ. Genetic mapping and characterization of the bleeding disorder in the fawn-hooded hypertensive rat. Thromb Haemost. 2003;89:1031–1042. [PubMed] [Google Scholar]
- Delay ER, Eddy MC, Eschle BK. Behavioral studies of umami: tales told by mice and rats. Ann N Y Acad Sci. 2009;1170:41–45. doi: 10.1111/j.1749-6632.2009.03933.x. [DOI] [PubMed] [Google Scholar]
- Delwiche JF, Buletic Z, Breslin PA. Covariation in individuals' sensitivities to bitter compounds: evidence supporting multiple receptor/transduction mechanisms. Percept Psychophys. 2001;63:761–776. doi: 10.3758/bf03194436. [DOI] [PubMed] [Google Scholar]
- DeSimone J, Ferrell F. Analysis of amiloride inhibition of chorda tympani taste response of rat to NaCl. Am J Physiol Regul Integr Comp Physiol. 1985;249:R52–R61. doi: 10.1152/ajpregu.1985.249.1.R52. [DOI] [PubMed] [Google Scholar]
- Dess NK. Saccharin's aversive taste in rats: evidence and implications. Neurosci Biobehav Rev. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- Di Nicolantonio R. Why does the spontaneously hypertensive rat have an exaggerated preference for sweet and salty solutions? An hypothesis. J Hypertens. 2004;22:1649–1654. doi: 10.1097/00004872-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Di Nicolantonio R, Kren V, Zidek V, Pravenec M. Salt preference of congenic strains derived from the spontaneously hypertensive rat. Physiol Behav. 2004;80:617–622. doi: 10.1016/j.physbeh.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Di Nicolantonio R, Mendelsohn FAO, Hutchinson JS. Sodium chloride preference of genetically hypertensive and normotensive rats. Am J Physiol Regul Integr Comp Physiol. 1983;245:R38–R44. doi: 10.1152/ajpregu.1983.245.1.R38. [DOI] [PubMed] [Google Scholar]
- Elliott EJ, Simon SA. The anion in salt taste: a possible role of paracellular pathways. Brain Res. 1990;535:9–17. doi: 10.1016/0006-8993(90)91817-z. [DOI] [PubMed] [Google Scholar]
- Formaker BK, Hill DL. An analysis of residual NaCl taste response after amiloride. Am J Physiol Regul Integr Comp Physiol. 1988;255:R1002–R1007. doi: 10.1152/ajpregu.1988.255.6.R1002. [DOI] [PubMed] [Google Scholar]
- Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- Gaillard D, Passilly-Degrace P, Besnard P. Molecular mechanisms of fat preference and overeating. Ann N Y Acad Sci. 2008;1141:163–175. doi: 10.1196/annals.1441.028. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–C1210. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Amit Z. Relative taste thresholds for ethanol, saccharin, and quinine solutions in three strains of rats nonselected for ethanol: a comparative study. Exp Clin Psychopharmacol. 2000;8:216–224. doi: 10.1037//1064-1297.8.2.216. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Bergeron N, Amit Z. Differences in the consumption of ethanol and flavored solutions in three strains of rats. Pharmacol Biochem Behav. 2000;65:357–362. doi: 10.1016/s0091-3057(99)00222-1. [DOI] [PubMed] [Google Scholar]
- Gregorova S, Divina P, Storchova R, Trachtulec Z, Fotopulosova V, Svenson KL, Donahue LR, Paigen B, Forejt J. Mouse consomic strains: exploiting genetic divergence between Mus m. musculus and Mus m. domesticus subspecies. Genome Res. 2008;18:509–515. doi: 10.1101/gr.7160508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. Altered responses to serotonergic agents in Fawn-Hooded rats. Pharmacol Biochem Behav. 1985;22:489–492. doi: 10.1016/0091-3057(85)90052-8. [DOI] [PubMed] [Google Scholar]
- Guenthner CJ, McCaughey SA, Tordoff MG, Baird JP. Licking for taste solutions by potassium-deprived rats: specificity and mechanisms. Physiol Behav. 2008;93:937–946. doi: 10.1016/j.physbeh.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol. 2008;99:1503–1514. doi: 10.1152/jn.00892.2007. [DOI] [PubMed] [Google Scholar]
- Halpern BP. Amiloride and vertebrate gustatory responses to NaCl. Neurosci Biobehav Rev. 1998;23:5–47. doi: 10.1016/s0149-7634(97)00063-8. [DOI] [PubMed] [Google Scholar]
- Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res. 1990;513:24–34. doi: 10.1016/0006-8993(90)91085-u. [DOI] [PubMed] [Google Scholar]
- Hill AE, Lander ES, Nadeau JH. Chromosome substitution strains: a new way to study genetically complex traits. Methods Mol Med. 2006;128:153–172. doi: 10.1385/1-59745-159-2:153. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs WJ, Beauchamp GK, Kare MR. Progress in animal flavor research. In: Bullard RW, editor. Flavor chemistry of animal foods. Washington (DC): American Chemical Society; 1978. pp. 1–20. [Google Scholar]
- Khan NA, Besnard P. Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim Biophys Acta. 2009;1791:149–155. doi: 10.1016/j.bbalip.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Kim UK, Breslin PA, Reed D, Drayna D. Genetics of human taste perception. J Dent Res. 2004;83:448–453. doi: 10.1177/154405910408300603. [DOI] [PubMed] [Google Scholar]
- Kondoh T, Mori M, Ono T, Torii K. Mechanisms of umami taste preference and aversion in rats. J Nutr. 2000;130:966S–970S. doi: 10.1093/jn/130.4.966S. [DOI] [PubMed] [Google Scholar]
- Kuijpers MH, Provoost AP, de Jong W. Development of hypertension and proteinuria with age in fawn-hooded rats. Clin Exp Pharmacol Physiol. 1986;13:201–209. doi: 10.1111/j.1440-1681.1986.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Laugerette F, Gaillard D, Passilly-Degrace P, Niot I, Besnard P. Do we taste fat? Biochimie. 2007;89:265–269. doi: 10.1016/j.biochi.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JR, Baker HJ. Historical foundations. In: Suckow MA, Weisbroth SH, Franklin CL, editors. The laboratory rat. New York: Academic Press; 2005. pp. 1–36. [Google Scholar]
- Liu L, Simon SA. Capsaicin and nicotine both activate a subset of rat trigeminal ganglion neurons. Am J Physiol. 1996;270:C1807–C1814. doi: 10.1152/ajpcell.1996.270.6.C1807. [DOI] [PubMed] [Google Scholar]
- Lu K, McDaniel AH, Tordoff MG, Li X, Beauchamp GK, Bachmanov AA, Vanderweele DA, Chapman CD, Dess NK, Huang L, et al. No relationship between sequence variation in protein coding regions of the Tas1r3 gene and saccharin preference in rats. Chem Senses. 2005;30:231–240. doi: 10.1093/chemse/bji019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush IE. The genetics of bitterness, sweetness, and saltiness in strains of mice. In: Wysocki CJ, Kare MR, editors. Chemical senses, volume 3. Genetics of perception and communication. New York: Marcel Dekker; 1991. pp. 227–241. [Google Scholar]
- Lush IE, Hornigold N, King P, Stoye JP. The genetics of tasting in mice. VII. Glycine revisited, and the chromosomal location of Sac and Soa. Genet Res. 1995;66:167–174. doi: 10.1017/s0016672300034510. [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, Desimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J Gen Physiol. 2005;125:587–600. doi: 10.1085/jgp.200509264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558:147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Desimone JA. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem Senses. 2005;30(Suppl 1):i42–i43. doi: 10.1093/chemse/bjh104. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Mizushige T, Yoneda T, Iwanaga T, Tsuzuki S, Inoue K, Fushiki T. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28:49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- Mattes RD. Is there a fatty acid taste? Annu Rev Nutr. 2009;29:305–327. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Cowley AW, Jr, Jacob HJ. Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am J Physiol Renal Physiol. 2007;293:F1905–F1914. doi: 10.1152/ajprenal.00012.2007. [DOI] [PubMed] [Google Scholar]
- McCaughey SA, Forestell CA, Tordoff MG. Calcium deprivation increases the palatability of calcium solution in rats. Physiol Behav. 2005;84:335–342. doi: 10.1016/j.physbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- McCaughey SA, Tordoff MG. Magnesium appetite in the rat. Appetite. 2002;38:29–38. doi: 10.1006/appe.2001.0443. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Milner P, Zucker I. Specific hunger for potassium in the rat. Psychon Sci. 1965;2:17–18. [Google Scholar]
- Mizushige T, Inoue K, Fushiki T. Why is fat so tasty? Chemical reception of fatty acid on the tongue. J Nutr Sci Vitaminol (Tokyo) 2007;53:1–4. doi: 10.3177/jnsv.53.1. [DOI] [PubMed] [Google Scholar]
- Moyer B, Zlotnick A, Hevezi P, Soto H, Lu M, Gao N, Servant G, Brust P, Williams M, Kalabat D, et al. Identification of TRPML3 (MCOLN3) as a salty taste receptor and use in assays for identifying taste (salty) modulators and/or therapeutics that modulate sodium transport, absorption or excretion and/or aldosterone and/or vasopressin production or release. 2009 U.S.P. Application 20090117563., Senomyx, May 7 2009. [Google Scholar]
- Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, Funakoshi M. Strain differences in amiloride inhibition of NaCl responses in mice, Mus musculus. J Comp Physiol [A] 1989;166:1–5. doi: 10.1007/BF00190204. [DOI] [PubMed] [Google Scholar]
- Ohsu T, Amino Y, Nagasaki H, Yamanaka T, Takeshita S, Hatanaka T, Maruyama Y, Miyamura N, Eto Y. Involvement of the calcium-sensing receptor in human taste perception. J Biol Chem. 2009;285:1016–1022. doi: 10.1074/jbc.M109.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Amiloride suppresses the sourness of NaCl and LiCl. Physiol Behav. 1996;60:1317–1322. doi: 10.1016/s0031-9384(96)00258-2. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Djouma E, Parsian A, Lawrence AJ. Depressive-like behavior and high alcohol drinking co-occur in the FH/WJD rat but appear to be under independent genetic control. Neurosci Biobehav Rev. 2007;31:103–114. doi: 10.1016/j.neubiorev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Parsian A. Behavioural features of alcohol-preferring rats: focus on inbred strains. Alcohol Alcohol. 1999;34:378–385. doi: 10.1093/alcalc/34.3.378. [DOI] [PubMed] [Google Scholar]
- PhysGen. PhysGen (Home Page) 2009. [Internet]. Available from: URL . http://pga.mcw.edu/ [Google Scholar]
- Provenza FD. Acquired aversions as the basis for varied diets of ruminants foraging on rangelands. J Anim Sci. 1996;74:2010–2020. doi: 10.2527/1996.7482010x. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Chemoreception for fat: do rats sense triglycerides directly? Appetite. 1992;18:193–206. doi: 10.1016/0195-6663(92)90197-e. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Role of olfaction in starch and oil preference. Am J Physiol Regul Integr Comp Physiol. 1993;265:R1404–R1409. doi: 10.1152/ajpregu.1993.265.6.R1404. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Chemosensory similarities among oils: does viscosity play a role? Chem Senses. 1994;19:155–168. doi: 10.1093/chemse/19.2.155. [DOI] [PubMed] [Google Scholar]
- Ramos Da Conceicao Neta ER, Johanningsmeier SD, McFeeters RF. The chemistry and physiology of sour taste—a review. J Food Sci. 2007;72:R33–R38. doi: 10.1111/j.1750-3841.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, et al. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Cleves M, Parsian A. Further genetic characterization of the fawn-hooded (FH/Wjd) rat, an animal model of comorbid depression and alcoholism. Psychiatr Genet. 2007;17:77–83. doi: 10.1097/YPG.0b013e328012d7c3. [DOI] [PubMed] [Google Scholar]
- Richter CP. Salt taste thresholds of normal and adrenalectomized rats. Endocrinol. 1939;24:367–371. [Google Scholar]
- Richter CP. Total self-regulatory functions in animals and human beings. Harvey Lect Ser. 1942–1943;38:63–103. [Google Scholar]
- Roitman MF, Bernstein IL. Amiloride-sensitive sodium signals and salt appetite: multiple gustatory pathways. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1732–R1738. doi: 10.1152/ajpregu.1999.276.6.R1732. [DOI] [PubMed] [Google Scholar]
- San Gabriel A, Uneyama H, Maekawa T, Torii K. The calcium-sensing receptor in taste tissue. Biochem Biophys Res Commun. 2009;378:414–418. doi: 10.1016/j.bbrc.2008.11.060. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Carbohydrate taste, appetite and obesity: an overview. Neurosci Biobehav Rev. 1987;11:131–153. [PubMed] [Google Scholar]
- Sclafani A. The sixth taste? Appetite. 2004;43:1–3. doi: 10.1016/j.appet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Clare RA. Female rats show a bimodal preference response to the artificial sweetener sucralose. Chem Senses. 2004;29:523–528. doi: 10.1093/chemse/bjh055. [DOI] [PubMed] [Google Scholar]
- Shao H, Burrage LC, Sinasac DS, Hill AE, Ernest SR, O'Brien W, Courtland HW, Jepsen KJ, Kirby A, Kulbokas EJ, et al. Genetic architecture of complex traits: large phenotypic effects and pervasive epistasis. Proc Natl Acad Sci U S A. 2008;105:19910–19914. doi: 10.1073/pnas.0810388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Ueda T, Ishida Y, Yamamoto T, Ugawa S. Acid-sensing ion channels in taste buds. Arch Histol Cytol. 2006;69:227–231. doi: 10.1679/aohc.69.227. [DOI] [PubMed] [Google Scholar]
- Shumake SA. Food preference behavior in birds and mammals. In: Bullard RW, editor. Flavor chemistry of animal foods. Washington (DC): American Chemical Society; 1978. pp. 21–42. [Google Scholar]
- Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O'Brien WE, Conti DV, Witte JS, Lander ES, et al. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- Stephens DW, Brown JS, Ydenberg RC. Foraging: behavior and ecology. Chicago (IL): University of Chicago Press; 2007. [Google Scholar]
- Stricker EM. Thirst and sodium appetite after colloid treatment in rats. J Comp Physiol Psychol. 1981;95:1–25. doi: 10.1037/h0077764. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Johnson AK. Thirst and salt appetite responses in young and old Brown Norway rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R317–R327. doi: 10.1152/ajpregu.00368.2002. [DOI] [PubMed] [Google Scholar]
- Tobach E, Bellin JS, Das DK. Differences in bitter taste perception in three strains of rats. Behav Genet. 1974;4:405–410. doi: 10.1007/BF01066160. [DOI] [PubMed] [Google Scholar]
- Tordoff MG. Gene discovery and the genetic basis of calcium appetite. Physiol Behav. 2008;94:649–659. doi: 10.1016/j.physbeh.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Lawler MP. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008;95:308–332. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of voluntary calcium intake, blood calcium, and bone mineral content. Physiol Behav. 2007a;91:632–643. doi: 10.1016/j.physbeh.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of water and sodium intake. Physiol Behav. 2007b;91:620–631. doi: 10.1016/j.physbeh.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Reed DR, Shao H. Calcium taste preferences: genetic analysis and genome screen of C57BL/6J x PWK/PhJ hybrid mice. Genes Brain Behav. 2008;7:618–628. doi: 10.1111/j.1601-183X.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Shao H, Alarcon LK, Margolskee RF, Mosinger B, Bachmanov AA, Reed DR, McCaughey SA. Involvement of T1R3 in calcium-magnesium taste. Physiol Genomics. 2008;34:338–348. doi: 10.1152/physiolgenomics.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigger SN, Pruitt KD, Fernandez-Suarez XM, Karolchik D, Worley KC, Maglott DR, Brown G, Weinstock G, Gibbs RA, Kent J, et al. What everybody should know about the rat genome and its online resources. Nat Genet. 2008;40:523–527. doi: 10.1038/ng0508-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verseput GH, Provoost AP, van Tol A, Koomans HA, Joles JA. Hyperlipidemia is secondary to proteinuria and is completely normalized by angiotensin-converting enzyme inhibition in hypertensive fawn-hooded rats. Nephron. 1997;77:346–352. doi: 10.1159/000190299. [DOI] [PubMed] [Google Scholar]
- Yongue BG, Myers MM. Further evidence for genetic independence of blood pressure and salt appetite in spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Hypertens A. 1989;11:25–33. doi: 10.3109/10641968909035288. [DOI] [PubMed] [Google Scholar]
- Young PT, Falk JL. The relative acceptability of sodium chloride solutions as a function of concentration and water need. J Comp Physiol Psychol. 1956;49:569–575. doi: 10.1037/h0047744. [DOI] [PubMed] [Google Scholar]
- Young PT, Greene JT. Relative acceptability of saccharine solutions as revealed by different methods. J Comp Physiol Psychol. 1953;46:295–298. doi: 10.1037/h0056212. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.