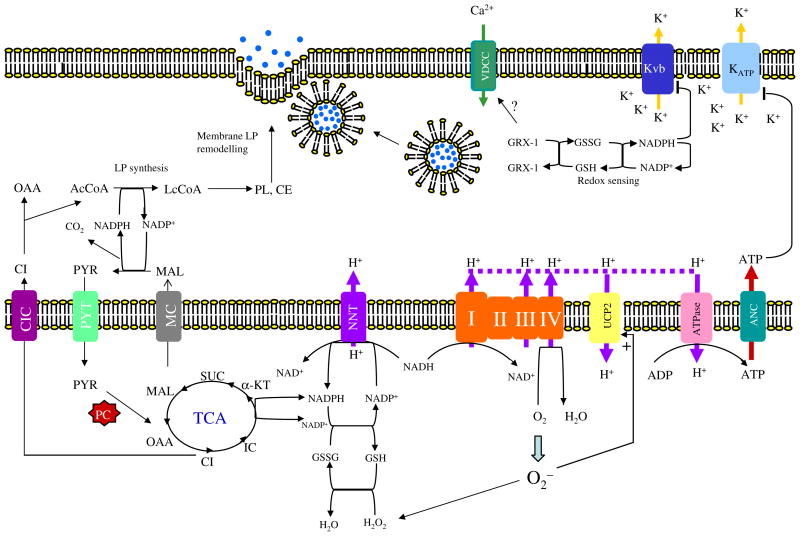
Fig. 3.
The role of lipogenesis and NADPH in insulin exocytosis. The NADPH produced from the pyruvate cycle is used for de novo fatty acid synthesis in pancreatic β-cells. This rapid lipogenesis alters cellular lipid composition, especially cholesteryl ester (CE) and phospholipids (PL) [52]. Modification of cholesterol content of the plasma membrane at the SNARE protein complex can potentiate insulin secretion [97]. The NADPH is also required for maintaining the cytoplasmic redox state through glutaredoxin GRX1, which acts as the molecular redox sensor [90]. The NADPH also regulates intracellular K+ by binding to its β-subunit (Kvβ), inhibiting K+ efflux through this channel and hence causing prolonged depolarisation of the intracellular membrane potential. The NADPH produced by NNT transhydrogenase is also required to detoxify ROS produced by respiratory chain activity [94]. The accumulation of ROS stimulates UCP2 activity, dissipating ATP production required to close the KATP channel. NADPH may also be transported to cytoplasm through the conversion of α-ketoglutarate to isocitrate catalysed by the mitochondrial NADP-dependent isocitrate dehydrogenase. NADPH can be reformed in the cytoplasm by cytosolic ME that converts the isocitrate-derived malate to pyruvate in the cytoplasm. ANC, adenosine nucleotide carrier; CE, cholesteryl ester; CI, citrate; IC, isocitrate; CIC, citrate/isocitrate carrier; GSSH, oxidised glutathione; GSH, reduced glutathione; α-KT, α-ketoglutarate; LP, lipid; MAL, malate; MC, malate carrier; OAA, oxaloacetate; PL, phospholipid; PYR, pyruvate; PYT, pyruvate transporter; SUC, succinate; VDCC, voltage-gate-dependent calcium channel