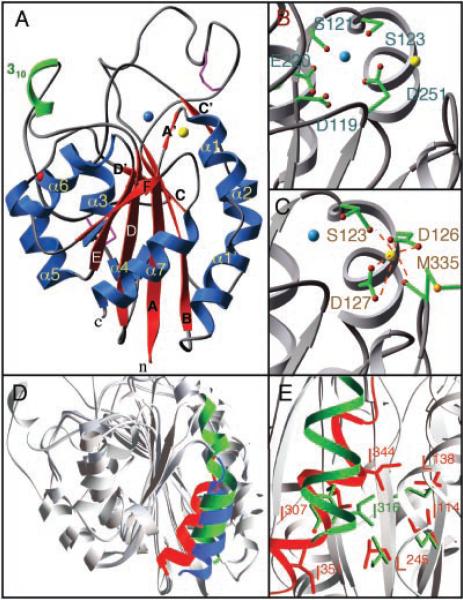
Fig. 3.
Structural features of the βA domain. (A) Ribbon drawing of the βA domain. Disulfides and the glycan are in purple and red, respectively. (B) Residues [single letter (41)] forming MIDAS that could participate in metal ion (blue) coordination. (C) Calcium (yellow) coordination at ADMIDAS. (D) Superimposition, based on the central β sheet, of the βA domain (minus the insertion in the B-C loop) and the “open” and “closed” forms of αA of CD11b (all gray). Shown are the α7-helices of the βA domain (red) and the “open” (blue) and “closed” (green) forms of αA of CD11b. (E) Coordination of SILEN in the βA domain (red) and in “closed” αA of CD11b (green). The βA domain SILEN residues [single letter, (41)] I114, L138, L245, and I307 (red) [equivalent to I135, L164, I236, and Y267, respectively, in αA of CD11b (green) (23)] coordinate I344 (equivalent in sequence to L312 in αA of CD11b). I351 (corresponding to I316 in αA of CD11b) is not coordinated in β3 SILEN but is partially exposed at the bottom of the structure.