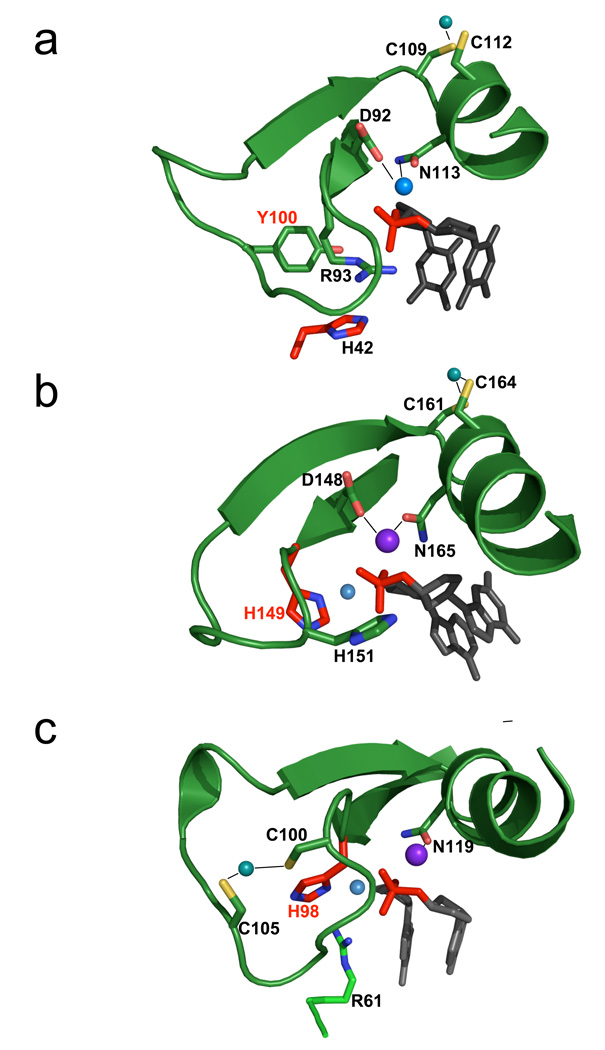
Figure 3. ββα-metal motifs and catalytic sites for HNH restriction and homing endonucleases.
The core catalytic motif, consisting of a two-stranded antiparallel beta-sheet and single alpha-helix, is shown in approximately the same orientation for (Panel a) PacI; (Panel b) Hpy99I and (Panel c) I-PpoI . The scissile phosphate is shown in red, flanked by its 5' and 3' nucleosides in gray. The histidine general base is colored and labeled with red, and the corresponding water nucleophile is a small light blue sphere. In all three catalytic sites, a single bound divalent metal ion (dark blue larger sphere) is coordinated by two asparagine/aspartate residues. An additional polar residue (participating in cleavage as a Lewis acid, whereby it stabilizes the phosphoanion transition state) is present in each catalytic site that is positioned to help satisfy the charge on the phosphate during cleavage (R93 for PacI; H151 for Hpy99I, and R61 for I-PpoI). Note that PacI displays a structural inversion in the positions of the histidine general base and the Lewis acid: His 42 is presented from a loop outside the ββα-metal motif, while R93 is found in the position that is usually occupied by an HNH histidine base. Finally, peripheral cysteine residues in all three ββα-metal motifs are involved in coordination of a structural zinc ion (C109 and C112 in PacI). However, the location of the bound zinc in the restriction endonucleases (panels a and b) differ from the homing endonuclease (panel c), indicating that these enzyme lineages may have diverged from a common ancestor prior to development of these metal binding sites. See also supplemental figure S3.