Abstract
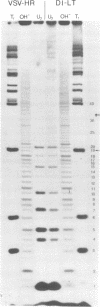
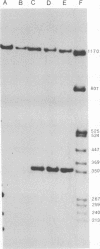
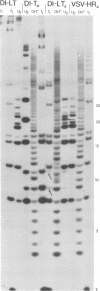
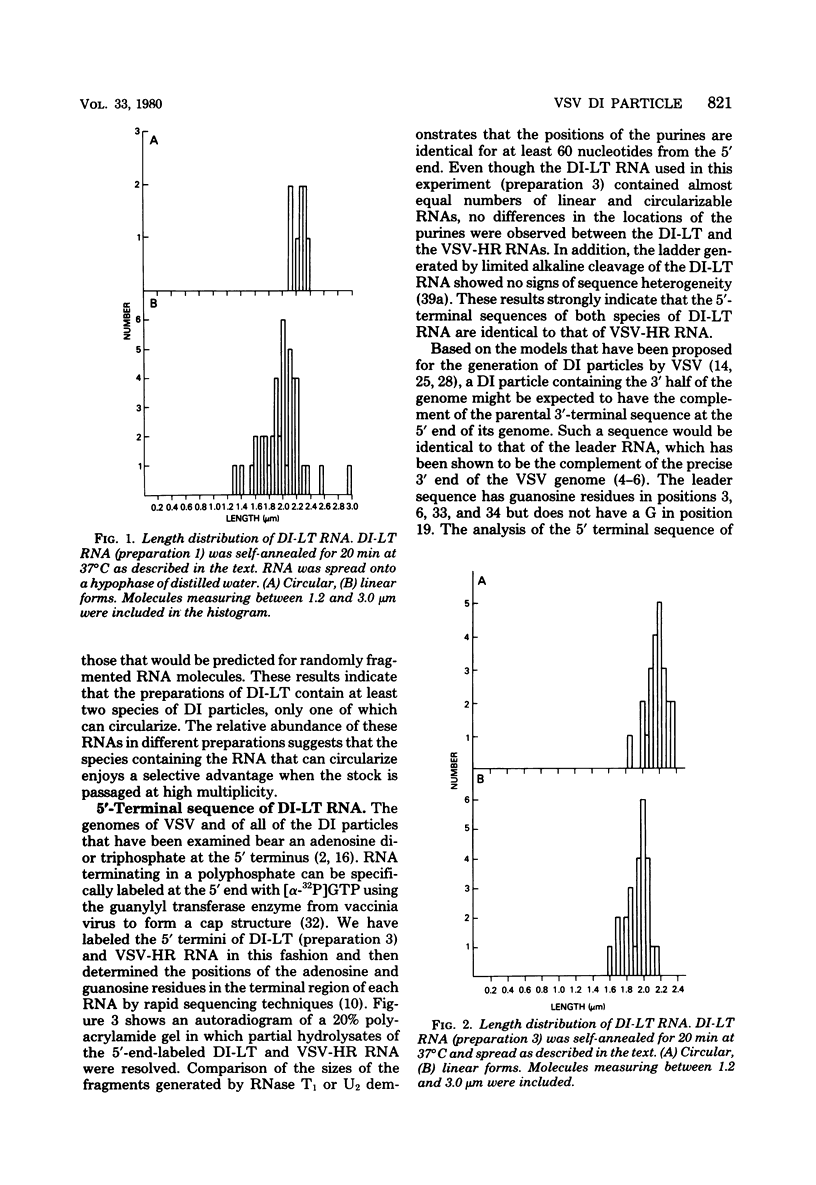
The genome structure of the long, truncated defective interfering particle derived from the heat-resistant strain of vesicular stomatitis virus has been examined. Stocks of this defective interfering particle are shown to contain several different species having information primarily from the 3' half of the vesicular stomatitis virus genome; the proportions of these components vary depending on the passage history of the stock. The two most abundant types have been identified and characterized. One has complementary 5' and 3' termini and consequently appears as a circular molecule when examined by electron microscopy. The other cannot circularize and remains linear. The circular forms are consistently 8 to 10% longer than the linear molecules. Rapid sequencing analyses reveal that both forms retain the 5' parental viral terminal sequence, but only the linear form retains the parental 3'-terminal sequence which is the complement of the 5' end. Hybridization experiments and electron microscopic analyses indicate that the linear form has retained 320 to 350 nucleotides of the 5' parental sequence and was probably generated by an internal deletion of the vesicular stomatitis virus genome.
Full text
PDF


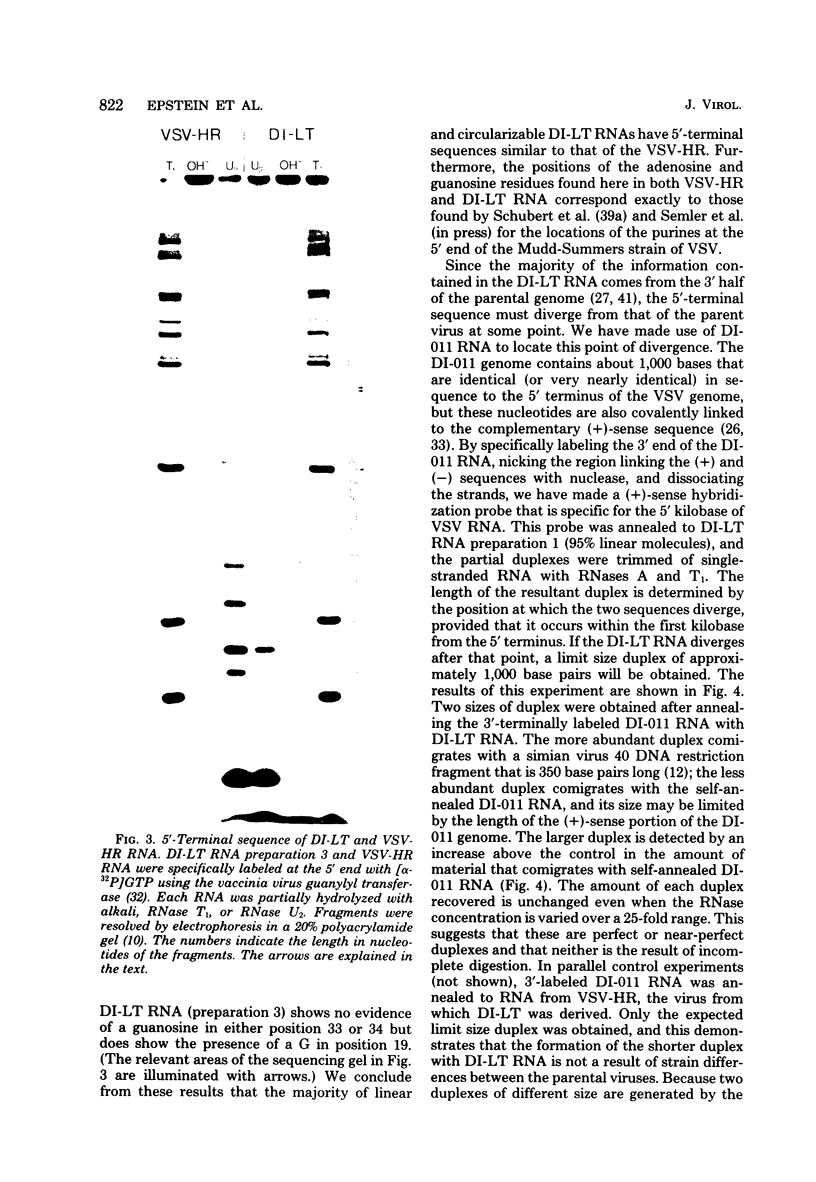

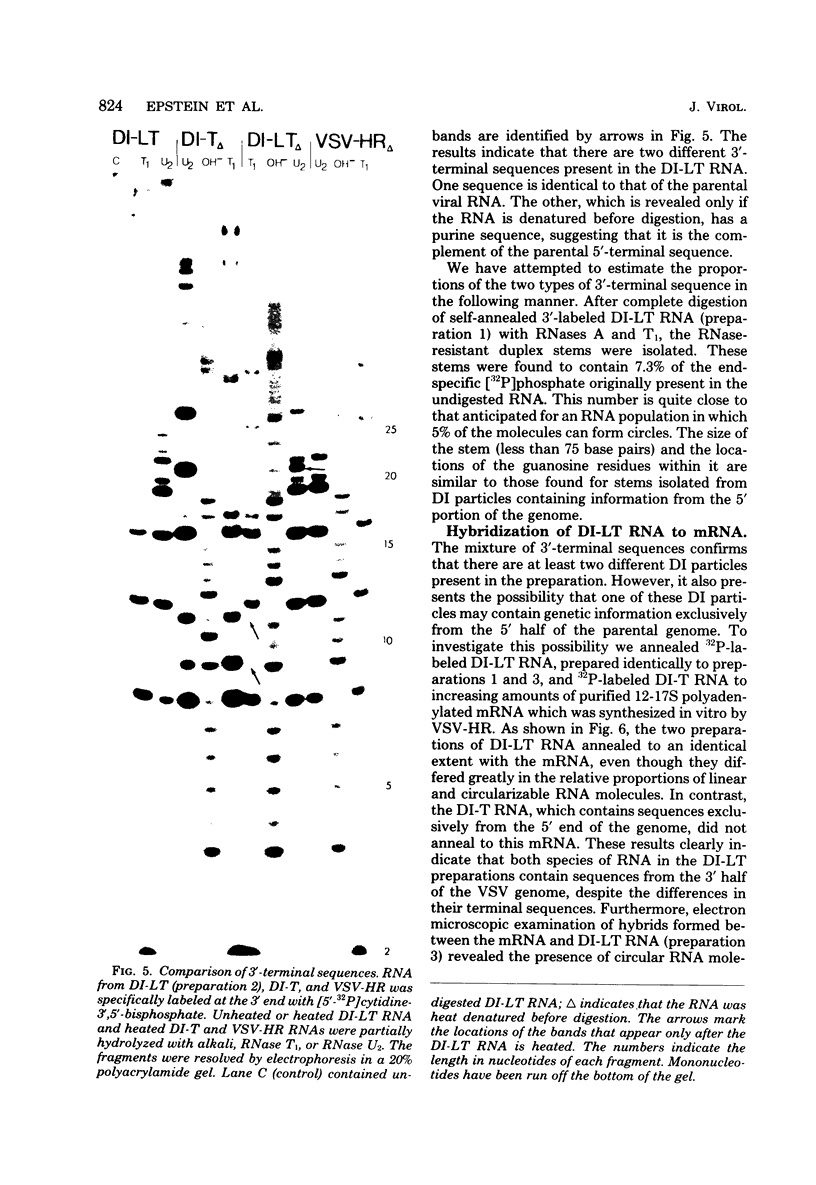
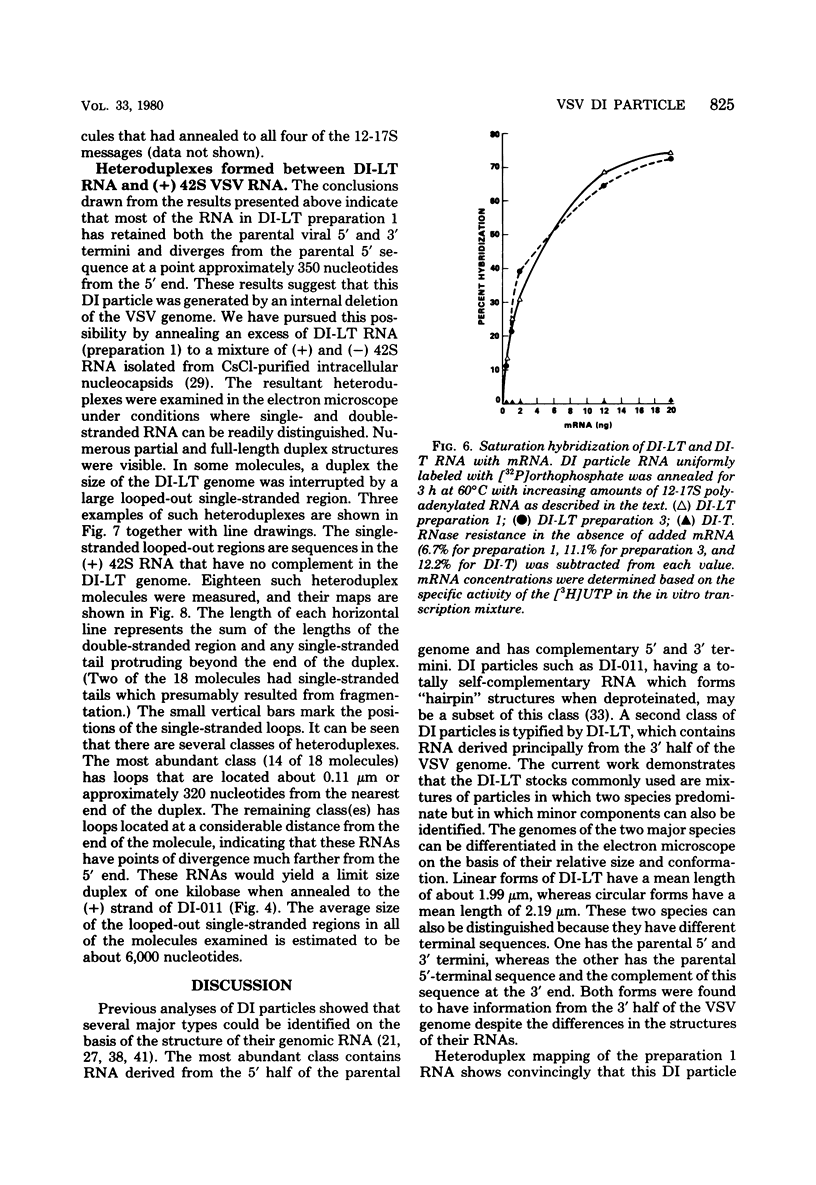
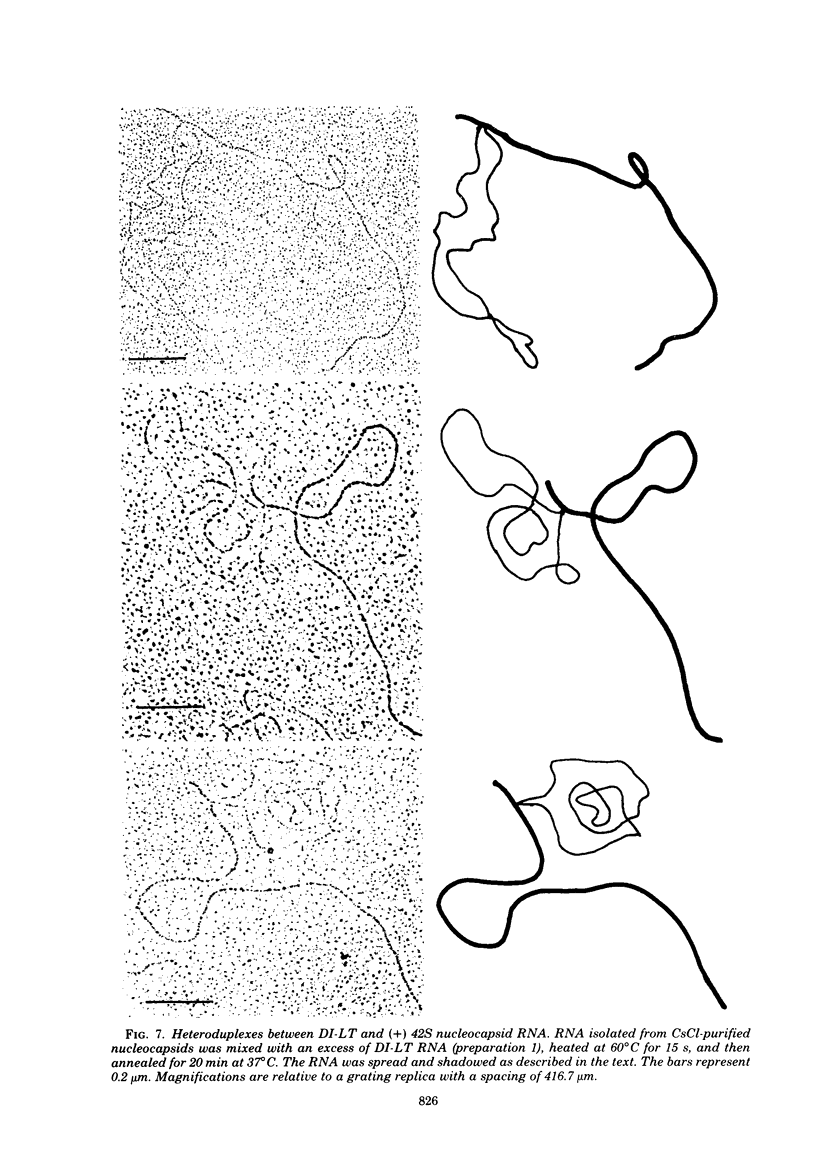



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Lazzarini R. A. Elementary aspects of autointerference and the replication of defective interfering virus particles. Virology. 1978 Jun 1;87(1):152–163. doi: 10.1016/0042-6822(78)90167-8. [DOI] [PubMed] [Google Scholar]
- Adler R., Banerjee A. K. Analysis of the RNA species isolated from defective particles of vesicular stomatitis virus. J Gen Virol. 1976 Oct;33(1):51–60. doi: 10.1099/0022-1317-33-1-51. [DOI] [PubMed] [Google Scholar]
- Chow J. M., Schnitzlein W. M., Reichmann M. E. Expression of genetic information contained in the RNA of a defective interfering particle of vesicular stomatitis virus. Virology. 1977 Apr;77(2):579–588. doi: 10.1016/0042-6822(77)90483-4. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Lazzarini R. A., Keene J. D., Banerjee A. K. In vitro synthesis of messenger RNA by a defective interfering particle of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 May;74(5):1884–1888. doi: 10.1073/pnas.74.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Darnell J. E. Relationship of chain transcription to poly(A) addition and processing of hnRNA in HeLa cells. Cell. 1974 Nov;3(3):255–264. doi: 10.1016/0092-8674(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Dingman C. W., Peacock A. C. Analytical studies on nuclear ribonucleic acid using polyacrylamide gel electrophoresis. Biochemistry. 1968 Feb;7(2):659–668. doi: 10.1021/bi00842a022. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. D., Lazzarini R. A. Replication of viral RNA by a defective interfering vesicular stomatitis virus particle in the absence of helper virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4387–4391. doi: 10.1073/pnas.74.10.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. D., Lazzarini R. A. The 5' terminal nucleotide of RNA from vesicular stomatitis virus defective interfering particles. Virology. 1977 Apr;77(2):863–866. doi: 10.1016/0042-6822(77)90508-6. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Allen R. Host function-dependent induction of defective interfering particles of vesicular stomatitis virus. J Virol. 1978 Jan;25(1):202–206. doi: 10.1128/jvi.25.1.202-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Glimp T., Clewley J. P., Bishop D. H. Studies on the generation of vesicular stomatitis virus (indiana serotype) defective interfering particles. Virology. 1978 Jan;84(1):142–152. doi: 10.1016/0042-6822(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Terminal sequences of vesicular stomatitis virus RNA are both complementary and conserved. J Virol. 1979 Oct;32(1):167–174. doi: 10.1128/jvi.32.1.167-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Weber G. H., Johnson L. D., Stamminger G. M. Covalently linked message and anti-message (genomic) RNA from a defective vesicular stomatitis virus particle. J Mol Biol. 1975 Sep 25;97(3):289–307. doi: 10.1016/s0022-2836(75)80042-8. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Reichmann M. E. The RNA of defective vesicular stomatitis virus particles in relation to viral cistrons. J Mol Biol. 1974 Jan 5;85(4):551–568. doi: 10.1016/0022-2836(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Lynch S., Kolakofsky D. Ends of the RNA within Sendai virus defective interfering nucleocapsids are not free. J Virol. 1978 Nov;28(2):584–589. doi: 10.1128/jvi.28.2.584-589.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J., Johnson L. D., Lazzarini R. A. Cell killing by viruses. V. Transcribing defective interfering particles of vesicular stomatitis virus function as cell-killing particles. Virology. 1977 Oct 1;82(1):242–246. doi: 10.1016/0042-6822(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Meyer J., Neuwald P. D., Lai S. P., Maizel J. V., Jr, Westphal H. Electron microscopy of late adenovirus type 2 mRNA hybridized to double-stranded viral DNA. J Virol. 1977 Mar;21(3):1010–1018. doi: 10.1128/jvi.21.3.1010-1018.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Characterization of snap-back RNAs in vesicular stomatitis defective interfering virus particles. J Gen Virol. 1978 Jan;38(1):21–34. doi: 10.1099/0022-1317-38-1-21. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978 Jan;38(1):35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Petric M., Prevec L. Vesicular stomatitis virus--a new interfering particle, intracellular structures, and virus-specific RNA. Virology. 1970 Aug;41(4):615–630. doi: 10.1016/0042-6822(70)90427-7. [DOI] [PubMed] [Google Scholar]
- Prevec L., Kang C. Y. Homotypic and heterotypic interference by defective particles of vesicular stomatitis virus. Nature. 1970 Oct 3;228(5266):25–27. doi: 10.1038/228025a0. [DOI] [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. The size and the cistronic origin of defective vesicular stomatitis virus particle RNAs in relation to homotypic and heterotypic interference. J Mol Biol. 1976 Mar 5;101(3):307–325. doi: 10.1016/0022-2836(76)90150-9. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- VON MAGNUS P. Propagation of the PR8 strain of influenza A virus in chick embryos. III. Properties of the incomplete virus produced in serial passages of undiluted virus. Acta Pathol Microbiol Scand. 1951;29(2):157–181. doi: 10.1111/j.1699-0463.1951.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Westphal H., Lai S. P. Quantitative electron microscopy of early adenovirus RNA. J Mol Biol. 1977 Nov 5;116(3):525–548. doi: 10.1016/0022-2836(77)90082-1. [DOI] [PubMed] [Google Scholar]