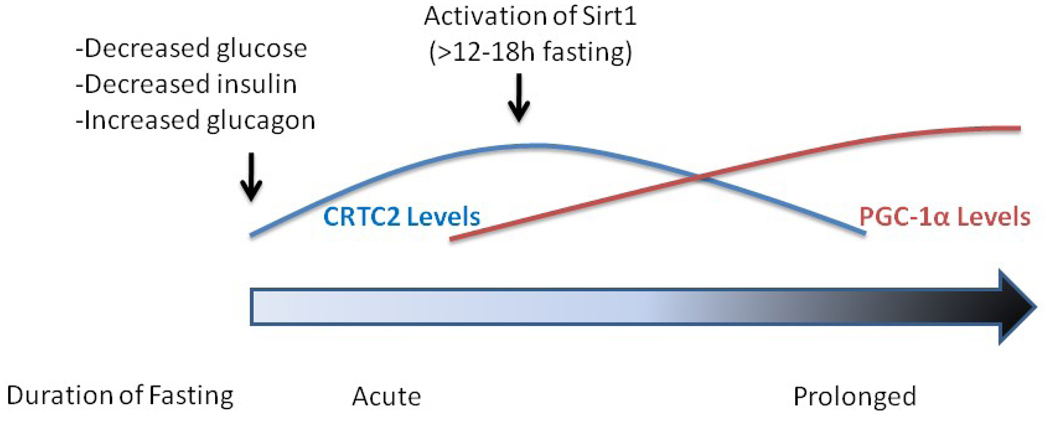
Figure 1.
A proposed theory for the temporal regulation of the two major transcriptional coactivators involved in hepatic gluconeogenesis during fasting. At the onset of fasting, circulating levels of glucose fall. This results in an increase in blood levels of glucagon and a decrease in insulin. This hormonal change produces an increase in both the levels and activity of the transcriptional coactivator CRTC2, which facilitates an increase in the expression of hepatic gluconeogenic genes. CRTC2 also increases the transcription of PGC-1α. With sustained fasting (>12–18h), Sirt1 becomes activated and deacetylates CRTC2. This event permits the ubiquitination of the protein by the E3 ligase COP1 and targets the enzyme for destruction [70]. The activation of Sirt1 also results in the deacetylation of PGC-1α, which is associated with an increase in the activity of this transcriptional coactivator.