Fig. 5. Regulation of SIRT1 activity at target gene promoters by nuclear NAD+- producing enzymes.
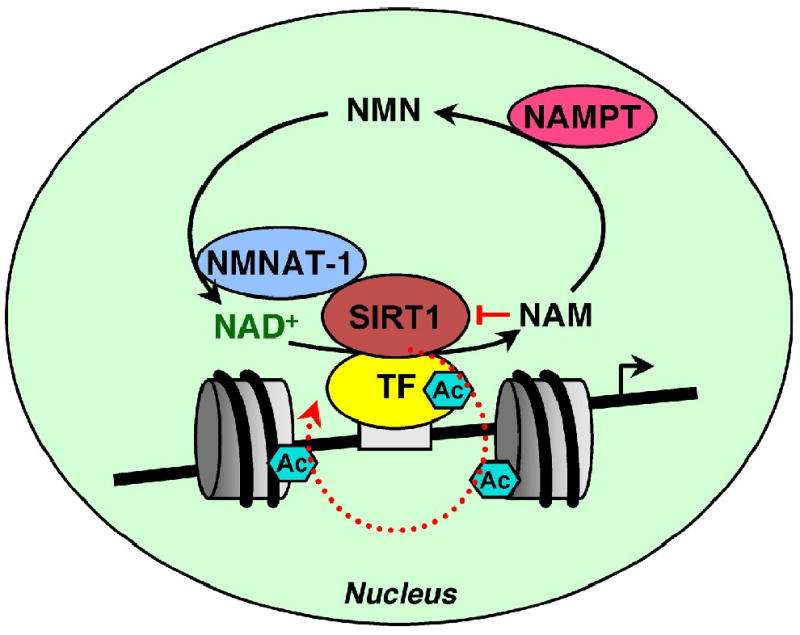
SIRT1 is recruited to target gene promoters and regulates the acetylation state of histones, transcription factors, and other chromatin-associated proteins in an NAD+-dependent manner. Nicotinamide (NAM), a byproduct of SIRT1-catalyzed deacetylation reactions, is a potent inhibitor of SIRT1 activity. NAMPT and NMNAT-1 constitute a nuclear NAD+ recycling pathway that utilizes NAM for NAD+ biosynthesis. NAM removal and NAD+ production by NAMPT and NMNAT-1 stimulate SIRT1 activity in the nucleus. In addition, NMNAT-1 interacts with SIRT1 and is recruited to SIRT1 target gene promoters. SIRT1-dependent recruitment of NMNAT-1 to chromatin may produce NAD+ for direct shuttling to SIRT1 to support its activity. TF, transcription factor. Ac, acetyl group.
