Abstract
The retinoic acid receptor-related orphan receptor α and γ (RORα [NR1F1] and RORγ [NR1F3]) are members of the nuclear hormone receptor superfamily. These 2 receptors regulate many physiological processes including development, metabolism and immunity. We recently found that certain oxysterols, namely the 7-substituted oxysterols, bound to the ligand binding domains (LBDs) of RORα and RORγ with high affinity, altered the LBD conformation and reduced coactivator binding resulting in suppression of the constitutive transcriptional activity of these two receptors. Here, we show that another oxysterol, 24S-hydroxycholesterol (24S-OHC), is also a high affinity ligand for RORα and RORγ (Ki ∼ 25 nM). 24S-OHC is also known as cerebrosterol due to its high level in the brain where it plays an essential role as an intermediate in cholesterol elimination from the CNS. 24S-OHC functions as a RORα/γ inverse agonist suppressing the constitutive transcriptional activity of these receptors in cotransfection assays. Additionally, 24S-OHC suppressed the expression of several RORα target genes including BMAL1 and REV-ERBα in a ROR-dependent manner. We also demonstrate that 24S-OHC decreases the ability of RORα to recruit the coactivator SRC-2 when bound to the BMAL1 promoter. We also noted that 24(S), 25-epoxycholesterol selectively suppressed the activity of RORγ. These data indicate that RORα and RORγ may serve as sensors of oxsterols. Thus, RORα and RORγ display an overlapping ligand preference with another class of oxysterol nuclear receptors, the liver X receptors (LXRα [NR1H3] and LXRβ [NR1H2]).
Keywords: Cholesterol, LXR, Lipid, REV-ERB, Circadian, Orphan Receptor
1. Introduction
Cholesterol is highly enriched in the brain relative to other organs where it is used as an essential component of membranes and the myelin sheath as well as for processes such as neuronal development, synaptogenesis, and learning and memory [1]. The blood brain barrier is impervious to peripheral lipoproteins and thus the cholesterol it requires must be synthesized de novo. This phenomenon also creates a problem for elimination of excess cholesterol, but a unique sterol transport pathway in the brain solves this. First, excess cholesterol is converted to the oxysterol 24S-hydroxycholesterol (24S-OHC) by the p450 cholesterol 24-hydroxylase (CYP46A1), an enzyme expressed in the brain. The vast majority of the total pool of 24S-OHC is produced in the brain providing a common name for this oxysterol – cerebrosterol. 24S-OHC is transported out of the CNS for elimination by the liver via high-density lipoprotein (HDL)-like particles composed of the primary apolipoprotein acceptor in the brain, apolipoprotein E (apoE), along with apoJ and apoA-1.
The liver X receptors, LXRα [NR1H3] and LXRβ [NR1H2] are nuclear hormone receptors that serve as receptors for 24S-OHC as well as other oxysterols [2-5]. The LXRs (predominately LXRβ in the brain) respond to elevated oxysterols and induce the expression of proteins such as ABCA1 and apoE that are required for cholesterol efflux and lipidation of HDL [6-8]. Given the role of cholesterol metabolism in Alzheimer's Disease (AD) etiology [9, 10], LXRs have been examined for their role in this disease. In a mouse model of AD (APP/PS1transgenic mouse) the loss of either LXRα or LXRβ results in increased amyloid plaque load [11]. Several laboratories have now shown that activation of LXR using synthetic agonists results in improvement of the AD phenotype in rodent models of the disease [12]-[13].
The retinoic acid receptor-related orphan receptors (RORs) are another group of nuclear receptors that have been implicated in regulation of cholesterol metabolism. RORα [NR1F1] is expressed in the brain and plays an essential role in development of the cerebellum as well as in regulation of the circadian rhythm [14]. RORγ [NR1F3] is best known for its role in regulation of T cell development [15], but both RORγ and RORα are expressed in the liver and likely play a role in regulation of glucose and lipid metabolism [16]. The crystal structure of RORα revealed cholesterol bound within the ligand binding domain (LBD) suggesting that this or a related sterol may be a natural functional ligand [17]. Recently, we found that certain oxysterols, in particular 7-oxygenated sterols, bind to RORα and RORγ with high affinity (∼20 nM Ki) and function as inverse agonists suppressing the endogenous transcriptional activity of these receptors [18]. Here, we show that the oxysterol 24S-OHC also binds to the LBDs of RORα and RORγ and regulates their transcriptional activity functioning as an inverse agonist.
2. Materials and Methods
2.1 Reagents
Oxysterols were obtained from Sigma (St. Louis, MO) or Steraloids (Newport, RI). Unless otherwise noted, oxysterols were used at a final concentration of 10 μM. Gal4-RORαLBD, Gal4-RORγLBD, pTrex-RORα, pTrex-RORγ, pGL-G6PC have been previously described [18]. The RORα adenoviral vector has also been previously described [18].
2.2 Cell Culture and Cotransfections
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum at 37 °C under 5% CO2. HepG2 cells were maintained and routinely propagated in minimum essential medium supplemented with 10% fetal bovine serum at 37 °C under 5% CO2. In experiments where lipids and sterols were depleted, cells were maintained on charcoal treated serum (10% fetal bovine serum) and treated with 7.5 μM lovastatin and 100 μM mevalonic acid. 24 h prior to transfection, HEK293 cells were plated in 96-well plates at a density of 15 × 103 cells/well. Transfections were performed using Lipofectamine™ 2000 (Invitrogen). 16 h post-transfection, the cells were treated with vehicle or compound. 24 h post-treatment, the luciferase activity was measured using the Dual-Glo™ luciferase assay system (Promega). The values indicated represent the means ± S.E. from four independently transfected wells. The experiments were repeated at least three times. Data was analyzed using GraphPad Prism software and IC50 values were determined by non-linear regression analysis.
2.3 cDNA synthesis and quantitative PCR
Total RNA extraction, cDNA synthesis, and real time quantitative PCR were performed as previously described [19] The primers used in quantitative PCR were: human REV-ERBα, TTCCGCTTCGGTGGAGCAGC (forward) and CCGGTTCTTCAGCACCAGAG (Reverse); human BMAL1, GTTCTTCTATTCTTGGTGAGAAC (forward) and ACAGCCATTGCTGCCTCATC (Reverse).
2.4 Chromatin Immunoprecipitation (ChIP)
ChIP-reChIP assays were performed as previously described [18, 20]. HepG2 cells were infected with adenovirus for 24 hours and then treated with vehicle or 24(S)-hydroxycholesterol, (10 μM) for another 24 hours. Re-ChIP assays were performed by using the kit from Active Motif®. Anti-FLAG (Sigma) was used to do the first immunoprecipitation for all the samples. The second immunoprecipitaion was performed by using anti-mouse IgG (Millipore), anti-RNA Pol II (Millipore) or anti-SRC2 (Bethyl Labs). The Bmal1 primers used in AGCGGATTGGTCGGAAAGTAG (forward) and GGCCTGACACTCACCGTG (Reverse).
2.5 Radioligand binding assay
RORα and RORγ LBDs were prepared as previously described [18]. Receptors were incubated with various concentrations of [3H]-25-hydroxycholesterol to determine the Kd values (RORα = 3.3±0.89 nM and RORγ = 5.1±0.71). Non-specific binding was defined in the absence of protein as well as excess of cold 25-hydroxycholesterol and were shown to be identical. The assays were terminated by rapid filtration through pre-soaked Whatman GF/B filters (0.5% PEI in PBS) in Multiscreen plates (Millipore) and were washed (3 × 1 ml) with ice-cold assay buffer. For the competition assay, various concentrations of sterols were incubated with receptor in the presence of 3 nM [3H]-25-hydroxycholesterol. Results were analyzed using GraphPad Prism software and the Ki was determined using the Cheng-Prusoff equation.
2.5 Statistical Analysis
Analysis was performed using GraphPad prism using a paired t test. Dose responses were analyzed using one-way ANOVA followed by a multiple comparison test. Experiments were performed on multiple occasions and the data normalized for analysis.
3. Results
Our previous studies demonstrated that 7α-OHC, 7β-OHC and 7-ketocholesterol (7-KC) bound directly to the LBDs of RORα and RORγ and regulated their transcriptional activity. These oxysterols functioned as inverse agonists repressing the constitutive transcriptional activation activity of the RORs [18].. Neither 22-OHC, 25-OHC, nor 27-OHC had an effect on the activity of the RORs [18], but we noticed that 24S-OHC (Figure 1A) behaved in a pattern similar to the 7-oxygenated sterols repressing the transcriptional activity of RORα and RORγ in a Gal4-ROR LBD cotransfection assay. We used the recently described synthetic ROR inverse agonist, T0901317 [20], as a control and compared the ability of 24S-OHC to suppress transcriptional activity of either Gal4-RORα LBD or Gal4-RORγ LBD in a cotransfection assay performed in HEK293 cells (Figures 1B and 1C). Treatment of cells with 10 μM 24S-OHC significantly reduced the transcriptional activity of either of the receptors by 30 to 40% (Figure 1). Using the same assay we then tested the dose-dependent response of the effect, and noted that for both receptors 24S-OHC demonstrated dose-dependent suppression of the transcriptional activity of both receptors with IC50s of 620 and 1300 nM for RORα and RORγ, respectively (Figures 2A and 2B). Maximal suppression was in the range of 30%, which is similar to what we previously noted for the 7-oxygenated sterols [18].
Figure 1.
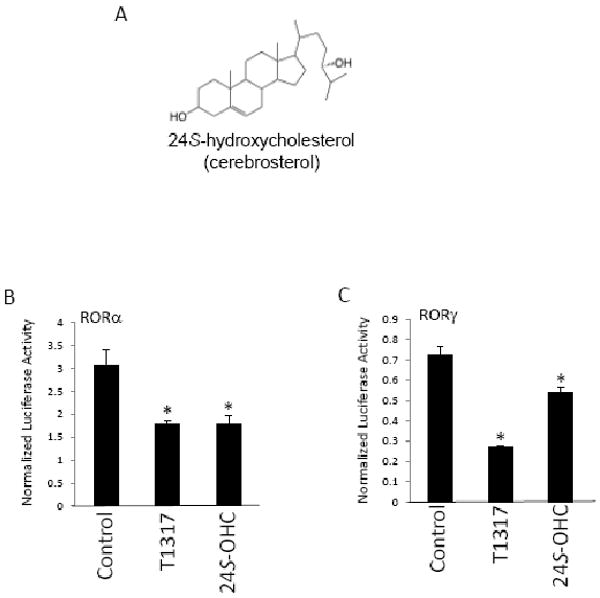
24S-hydroxycholesterol suppresses the transcriptional activity of the RORα and RORγ LBDs. (A) Chemical structure of 24S-hydroxycholesterol. (B) Cotransfection assay in HEK293 cells illustrating the ability of 10 μM 24S-OHC to suppress the transcriptional activation activity of either Gal4-RORα LBD or Gal4-RORγ LBD. T0901317 (T1317) functions as an inverse agonist for both RORs and is used as a positive control. * indicates p<0.05.
Figure 2.
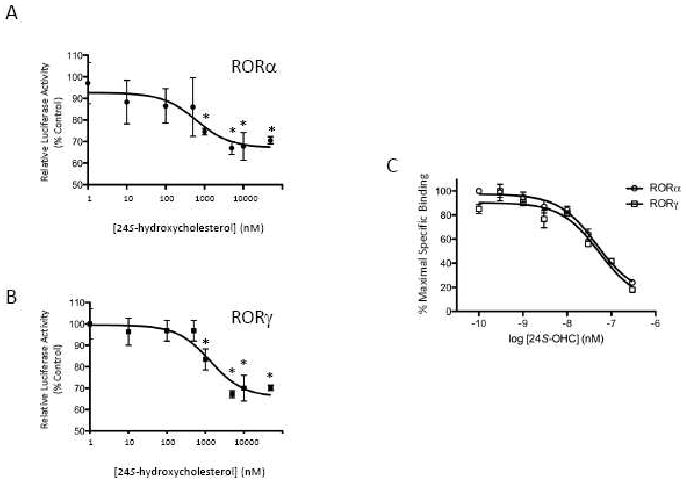
24S-hydroxycholesterol suppresses the transcriptional activity of the RORα and RORγ LBDs in a dose-dependent manner. Cotransfection assay in HEK293 cells illustrating the ability 24S-OHC to suppress the transcriptional activation activity of either Gal4-RORα LBD (A) or Gal4-RORγ LBD (B). (C) 24S-hydroxycholesterol is a RORα and RORγ ligand. Results from a competitive radioligand binding assay are shown where [3H]25-OHC was used as the radioligand.* indicates p<0.05.
We performed a competition radioligand binding assay using bacterially expressed RORα and RORγ LBD and radiolabeled 25-OHC as the tracer. This assay was previously used to demonstrate direct binding of 7α-OHC, 7β-OHC, and 7-KC to RORα and RORγ [18]. We found that 24S-OHC effectively competed for [3H]-25-OHC binding to either RORα or RORγ (Figure 2C) with Ki values that were virtually identical for both receptor subtypes (RORα = 27 nM; RORγ = 25 nM). These values are very similar to the Ki values found for the 7-oxygenated sterols that ranged from 12 – 31 nM [18].
We examined the ability of 24S-OHC to modulate the activity of full-length RORα and RORγ using a cotransfection assay where HEK293 cells were transfected with either of the receptors and a reporter vector with the Bmal1 promoter directing the expression of luciferase. Bmal1 is a well-characterized ROR target gene with ROR response elements (ROREs) within its proximal promoter [21]. As illustrated in Figures 3A and 3B, treatment of the cotransfected cells with 10 μM 24S-OHC led to significant suppression of the Bmal1 driven reporter gene expression. The suppression was modest (20-40%), but was statistically significant and reminiscent of the “partial” inverse agonist activity we have previously observed for oxysterol ligands of the RORs [18]. The glucose-6-phosphatase (G6Pase) gene is also a RORα target gene (containing a RORE) providing a role for ROR in regulation of glucose metabolism [22]. RORα can activate transcription of a G6Pase promoter driven reporter gene [18, 22]. and as such, we performed a cotransfection assay with this reporter along with RORα in the presence and absence of 10 μM 24S-OHC. The oxysterol suppressed expression of the reporter by approximately 30% consistent with its ability to suppress RORα activity (Figure 3C).
Figure 3.
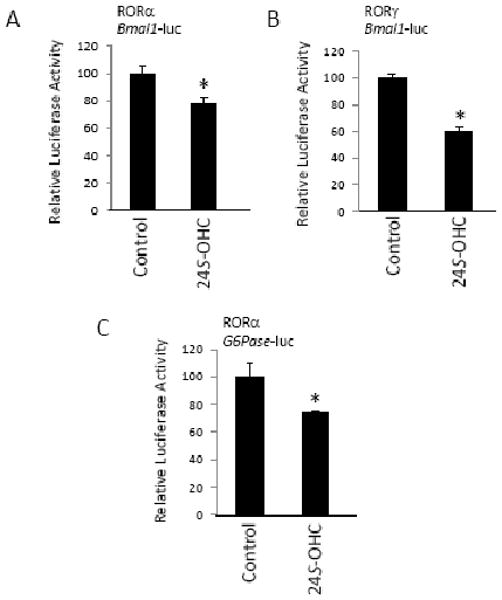
24S-hydroxycholesterol suppresses the transcriptional activity of full-length RORα and RORγ. Cotransfection assay in HEK293 cells illustrating the ability of 10 μM 24S-OHC to suppress the transcriptional activation activity of either RORα (A) or RORγ (B) driving expression of a BMAL1 promoter – luciferase construct. (C) Cotransfection assay in HepG2 cells illustrating the ability of 10 μM 24S-OHC to suppress the transcriptional activation activity of RORα driving expression of a G6Pase promoter – luciferase construct. * indicates p<0.05.
The ability of 24S-OHC to regulate expression of RORα target genes was also evaluated. Both the BMAL1 and REV-ERBα genes are direct RORα target genes [23-25]. Treatment of HepG2 cells with 24S-OHC resulted in a decrease in both of these RORα target genes (Figures 4A and 4B). BMAL1 mRNA expression decreased 38% while REV-ERBα expression decreased 33%. These effects were dependent on RORα expression since knock-down of RORα using siRNA (Fig. 4C) [18] blocked the ability of 24S-OHC to suppress expression of either target gene (Figures 4A and 4B). Interestingly, basal expression of both BMAL1 and REV-ERBα decreased when RORα expression was knocked down, suggesting that the constitutive activity of RORα helps to maintain the basal expression of these two genes. Similar effects were also noted with G6Pase [18]. In order to determine if 24S-OHC modulates the ability of RORα to recruit a coactivator protein we used a ChIP-reChIP assay [26]. This format was used so that we could monitor the amount of the transcriptional coactivator, SRC-2, bound to RORα while this receptor occupied the BMAL1 promoter. SRC-2 was selected since it has been shown to be a critical coactivator for RORα function [22]. As shown in Figure 4D, equivalent amounts of RORα are detected in the absence (DMSO) or presence of 24S-OHC. However, in the reChIP assay the amount of SRC-2 recruited is reduced (∼50%) in the 24S-OHC lane versus the DMSO control indicating that 24S-OHC treatment reduces the ability of RORα to recruit this coactivator.
Figure 4.
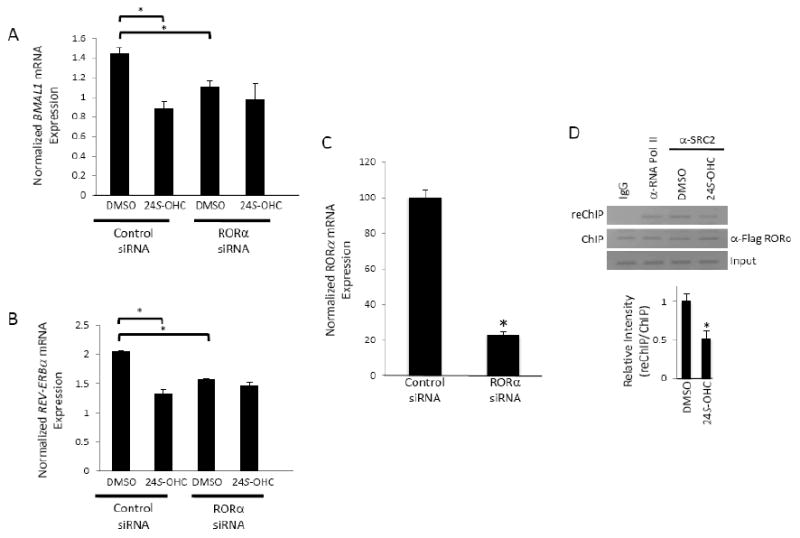
24S-hydroxycholesterol modulates the expression of RORα target genes. 24S-hydroxycholesterol (10 μM) suppresses the expression of BMAL1 (A) and REV-ERBα (B) mRNA in a RORα dependent manner in HepG2 cells. (C) Expression of RORα following treatment of cells with siRNA targeting RORα. Control cells received scrambled siRNA. (D) ChIP-reChIP assay illustrating the ability of 24S-OHC to decrease SRC-2 recruitment to RORα occupying the BMAL1 promoter. The graph below the gel indicates the average band intensity from three experiments. IgG is used as a negative control and α-RNA pol II antibody is used as a positive control. * indicates p<0.05.
Previously we reported that 7-oxygenated sterols functioned as RORα/γ partial inverse agonists while 22-OHC, 25-OHC, and 27-OHC did not affect the function of these constitutively active receptors. Based on the activity we observed with 24S-OHC we decided to test additional structurally related oxysterols. We first tested the unnatural epimer of 24S-OHC, 24R-OHC. Both stereoisomers have been shown to activate LXRα and LXRβ although 24R-OHC is less potent than 24S-OHC [3, 5]. When we tested 24R-OHC at a single dose of 10 μM in a Gal4-RORα/γ LBD cotransfection assay we noted a significant decrease in the constitutive activity of RORγ, but no effect on RORα (Fig. 5A & 5B). We performed a dose-response in the cotransfection assay with the Gal4-RORγ LBD construct and as shown in Fig. 4C, there was a dose-dependent suppression of transcriptional activity (IC50=90 nM). We then performed a radioligand binding assay with RORγ and confirmed that 24R-OHC bound to the receptor with a Ki of 102 nM. We also tested the activity of 24(S),25 epoxycholesterol (24,25-epoC), an oxysterols that has a well-characterized effect on cholesterol biosynthesis, which is synthesized at relatively high levels in the liver (found at micromolar concentrations) [27], is produced by neurons and astrocytes [28] and found at very high levels in the developing brain. 24,25-epoC is an effective ligand that activates both LXRα and LXRβ [3, 5]. As shown in Fig. 6A, this oxysterol suppresses the activity of RORγ without having any significant effect on RORα (Gal4-LBD cotransfection assay). 24,25-epoC dose-dependently suppressed RORγ activity (IC50 = 280 nM) as well as bound directly to the RORγ LBD with a Ki of 20 nM.
Figure 5.
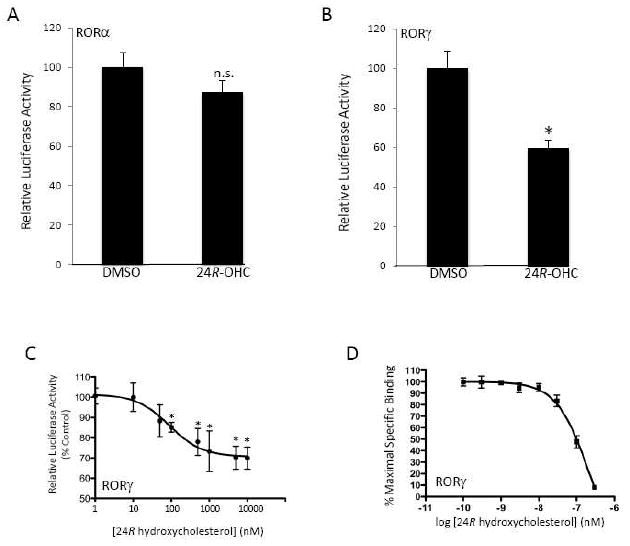
24R-hydroxycholesterol suppresses the transcriptional activity of the RORγ LBD. . (A) Cotransfection assay in HEK293 cells illustrating the inability of 10 μM 24S-OHC to affect the transcriptional activation activity of Gal4-RORα LBD. (B) Cotransfection assay in HEK293 cells illustrating the inability of 10 μM 24S-OHC to suppress the transcriptional activation activity of Gal4-RORγ LBD. (C) Cotransfection assay in HEK293 cells illustrating the ability 24S-OHC to suppress the transcriptional activation activity of Gal4-RORγ LBD dose-dependently. (D) Results from a competitive radioligand binding assay are shown where [3H]25-OHC was used as the radioligand and 24R-OHC was used as a competitor. * indicates p<0.05.
Figure 6.
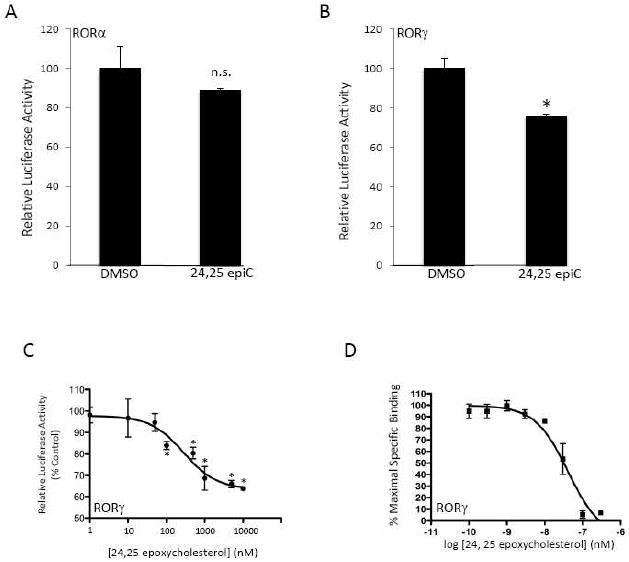
24(S),25-epoxycholesterol suppresses the transcriptional activity of the RORγ LBD.. (A) Cotransfection assay in HEK293 cells illustrating the inability of 10 μM 24S-OHC to affect the transcriptional activation activity of Gal4-RORα LBD. (B) Cotransfection assay in HEK293 cells illustrating the inability of 10 μM 24(S),25-epoxycholesterol to suppress the transcriptional activation activity of Gal4-RORγ LBD. (C) Cotransfection assay in HEK293 cells illustrating the ability 24(S),25-epoxycholesterol to suppress the transcriptional activation activity of Gal4-RORγ LBD dose-dependently. (D) Results from a competitive radioligand binding assay are shown where [3H]25-OHC was used as the radioligand and 24(S),25-epoxycholesterol was used as a competitor. * indicates p<0.05.
4. Discussion
Previously, we demonstrated that 7-substituted oxysterols function as ligands for RORα and RORγ by binding to their LBDs, altering the conformation of the LBD, and decreasing the ability of the receptors to recruit coactivator and thus decreasing their transcriptional activity [18]. These effects were specific since other oxysterols such as 22-OHC, 25-OHC, and 27-OHC were unable to affect the activity of either RORα or RORγ. Here we show that 24S-OHC also serves as a functional RORα/RORγ ligand by binding to the LBDs with high affinity and altering the ability of the receptor to recruit SRC-2 on a target gene promoter and thus reducing their transcriptional activity. Given the role of RORα in development of regions of the brain and regulation of the circadian behavior as well as the importance of 24S-OHC as the major oxysterol in the brain, we find these results intriguing. The brain converts cholesterol enzymatically to 24S-OHC via CYP46A1-dependent oxidation of the sterol so that it can be effluxed from the brain for elimination by the liver. The liver expresses significant amounts of both RORα and RORγ, thus it is possible that 24S-OHC levels in the liver may also regulate the activity of these two nuclear receptors.
Plasma levels of 24S-OHC in normal individuals range from 50 nM to 350 nM and are found to be elevated in patients with AD [29, 30]. Patients treated with 3-hydroxy-3methylglutaryl (HMG)-CoA reductase inhibitors (statins) for dyslipidemia have been shown to have lower incidence of AD [31, 32] and statins have been demonstrated to lower 24S-OHC plasma levels in patients with AD [33]. Interestingly, plasma 24S-OHC levels are highest when young and decrease significantly with aging in humans [30]. In fact, the average plasma levels for children aged 1-5 years old is near 1 μM whereas the average levels of adults aged 19-50 years old is approximately 190 nM [30]. This 5-fold difference may reflect an important role for this oxysterol during brain development and our results suggesting that 24S-OHC is a ligand for RORα indicates that the activity of RORα may be modulated during this time.
24S-OHC also functions as a ligand for LXRα/β where it acts as an agonist to increase the transcriptional activity of the receptors [2-5], while this same oxysterol acts as an inverse agonist of RORα and RORγ to repress their transcriptional activity. 24S-OHC displays similar binding affinities for LXRα/β [5] as it does for RORα/γ and additionally, the potency for activation of the LXRs [3, 5] is similar to the potency for suppression of the RORs. Interestingly, we have also observed a similar pattern of ligand preference between LXRs and RORs with respect to synthetic ligands. We found that the widely used LXR agonist, T0901317, also binds directly to the RORα and RORγ LBDs and inhibits their transcriptional activity functioning as an inverse agonist [20]. Thus, it appears to be common that ligands, even from unique chemotypes, may bind to both LXRs and RORs and function as an agonist on LXR and inverse agonist on ROR. However, it should be noted that although the RORs and LXRs display overlapping ligand selectivity there are clear examples of preferential ligands. For example, 7α-OHC and 7-ketocholesterol are high affinity RORα/γ ligands [18] but display no affinity for the LXRs [5].
LXR agonists such as T0901317 and GW3965 have been shown to have beneficial effects on plaque formation and cognition in mouse models of AD[11-13, 34] and the cross-reactivity of LXR ligands with ROR brings into question whether some of the efficacy of these ligands might be mediated by ROR. Genetic evidence is supportive of LXR playing a very important role in the efficacy exemplified by the observation that loss of either LXRα or LXRβ expression in a mouse model of AD (APP/PS1transgenic mouse) results in increased amyloid plaque load [11]. Currently, it is unclear whether the ROR activity would help, hinder or have no effect on the efficacy of T0901317 in the AD models.
In our analysis of specificity of RORα/γ for other oxysterols, we found that 24R-OHC and 24,25-epoC both regulated RORγ selectively. This suggests that RORγ may be somewhat more promiscuous in terms of its oxysterol selectivity. The high levels of RORγ and 24,25-epoC in the liver suggests that this oxysterol may be modulating its RORγ's activity.
In summary, we have found that 24S-OHC binds to the LBDs of RORα and RORγ and suppresses their ability to activate transcription. These data suggest that ROR may be a target for this sterol that plays an important role in cholesterol transport in the CNS.
Acknowledgments
The efforts of P.R.G. were supported by the National Institutes of Health (NIH) Molecular Library Screening Center Network (MLSCN) grant U54MH074404 (Hugh Rosen, Principal Investigator). This work was also supported by NIH grants DK080201, NS066417, NS067589 (T.P.B.) and GM084041 (P.R.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Current Opinion in Lipidology. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Chen WL, Chen GX, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metabolism. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the Nuclear Receptor LXR by Oxysterols Defines a New Hormone Response Pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 4.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 5.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXR alpha and LXR beta. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitney KD, Watson MA, Collins JL, Benson WG, Stone TM, Numerick MJ, Tippin TK, Wilson JG, Winegar DA, Kliewer SA. Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Mol Endocrinol. 2002;16:1378–1385. doi: 10.1210/mend.16.6.0835. [DOI] [PubMed] [Google Scholar]
- 7.Laffitte BA, Repa JJ, Joseph SB, Wilpiltz DC, Kast HR, Mangelsdorf DJ, Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang Y, Lin SZ, Beyer TP, Zhang YY, Wu X, Bales KR, DeMattos RB, May PC, Li SYD, Jiang XC, Eacho PI, Cao GQ, Paul SM. A liver X receptor and retinoid X receptor heterodimer mediates apolipoprotein E expression, secretion and cholesterol homeostasis in astrocytes. Journal of Neurochemistry. 2004;88:623–634. doi: 10.1111/j.1471-4159.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 9.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer's disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 10.Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J Neurochem. 2007;102:1727–1737. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- 11.Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc Natl Acad Sci U S A. 2007;104:10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, Ring RH, Kirksey Y, Aschmies S, Xu J, Kubek K, Hirst WD, Gonzales C, Chen Y, Murphy E, Leonard S, Vasylyev D, Oganesian A, Martone RL, Pangalos MN, Reinhart PH, Jacobsen JS. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol Cell Neurosci. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Koldamova RP, Lefterov IM, Staufenbiel M, Wolfe D, Huang S, Glorioso JC, Walter M, Roth MG, Lazo JS. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease. J Biol Chem. 2005;280:4079–4088. doi: 10.1074/jbc.M411420200. [DOI] [PubMed] [Google Scholar]
- 14.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109:S57–S66. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 16.Kang HS, Angers M, Beak JY, Wu X, Gimble JM, Wada T, Xie W, Collins JB, Grissom SF, Jetten AM. Gene expression profiling reveals a regulatory role for ROR alpha and ROR gamma in phase I and phase II metabolism. Physiological Genomics. 2007;31:281–294. doi: 10.1152/physiolgenomics.00098.2007. [DOI] [PubMed] [Google Scholar]
- 17.Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. X-ray structure of the hROR alpha LBD at 1.63 angstrom: Structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of ROR alpha. Structure. 2002;10:1697–1707. doi: 10.1016/s0969-2126(02)00912-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RA, Stayrook KR, Zhang X, Novick S, Chalmers MJ, Griffin PR, Burris TP. Modulation of RORalpha and RORgamma activity by 7-oxygenated sterol ligands. Journal of Biological Chemistry. 2010;285:5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERB[alpha] and REV-ERB[beta] Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez R, Burris TP, Griffin PR. The benzenesulfonamide T0901317 is a novel ROR{alpha}/{gamma} Inverse Agonist. Molecular Pharmacology. 2010;77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burris TP. Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock. Mol Endocrinol. 2008;22:1509–1520. doi: 10.1210/me.2007-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopra AR, Louet JF, Saha P, An J, DeMayo F, Xu JM, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW. Absence of the SRC-2 Coactivator Results in a Glycogenopathy Resembling Von Gierke's Disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delerive P, Chin WW, Suen CS. Identification of Reverb alpha as a novel ROR alpha target gene. Journal of Biological Chemistry. 2002;277:35013–35018. doi: 10.1074/jbc.M202979200. [DOI] [PubMed] [Google Scholar]
- 24.Raspe E, Mautino G, Duval C, Fontaine C, Duez H, Barbier O, Monte D, Fruchart J, Fruchart JC, Staels B. Transcriptional regulation of human Rev-erb alpha gene expression by the orphan nuclear receptor retinoic acid-related orphan receptor alpha. Journal of Biological Chemistry. 2002;277:49275–49281. doi: 10.1074/jbc.M206215200. [DOI] [PubMed] [Google Scholar]
- 25.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, Fitzgerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 27.Spencer TA, Gayen AK, Phirwa S, Nelson JA, Taylor FR, Kandutsch AA, Erickson SK. 24(S),25-Epoxycholesterol - Evidence Consistent with a Role in the Regulation of Hepatic Cholesterogenesis. Journal of Biological Chemistry. 1985;260:3391–3394. [PubMed] [Google Scholar]
- 28.Wong J, Quinn CM, Guillemin G, Brown AJ. Primary human astrocytes produce 24(<i>S</i>),25-epoxycholesterol with implications for brain cholesterol homeostasis. Journal of Neurochemistry. 2007;103:1764–1773. doi: 10.1111/j.1471-4159.2007.04913.x. [DOI] [PubMed] [Google Scholar]
- 29.Lutjohann D, Papassotiropoulos A, Bjorkhem I, Locatelli S, Bagli M, Oehring RD, Schlegel U, Jessen F, Rao ML, von Bergmann K, Heun R. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. Journal of Lipid Research. 2000;41:195–198. [PubMed] [Google Scholar]
- 30.Bretillon L, Lutjohann D, Stahle L, Widhe T, Bindl L, Eggertsen G, Diczfalusy U, Bjorkhem I. Plasma levels of 24S-hydroxycholesterol reflect the balance between cerebral production and hepatic metabolism and are inversely related to body surface. Journal of Lipid Research. 2000;41:840–845. [PubMed] [Google Scholar]
- 31.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 32.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Archives of Neurology. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 33.Vega GL, Weiner MF, Lipton AM, von Bergmann K, Lutjohann D, Moore C, Svetlik D. Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Archives of Neurology. 2003;60:510–515. doi: 10.1001/archneur.60.4.510. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Q, Lee CYD, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of A beta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


