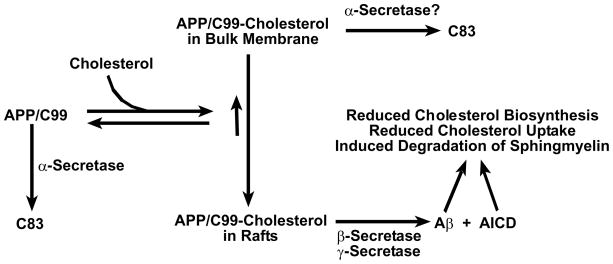
Figure 3.
Working model for how cholesterol binding to C99/APP is related to the amyloidogenic and non-amyloidogenic pathways for processing APP and for how the amyloidogenic pathway may be linked to cellular cholesterol reduction. Given that the transmembrane product of α-secretase cleavage of APP (C83) contains the sequence motif believed to be central to cholesterol binding by C99/APP (see Figure 1), C83 probably also binds cholesterol. In this case, elevated cholesterol levels would also result in raft localization of C83, subsequent γ-secretease cleavage of which would then release AICD and an apparently harmless peptide known as p3. Whether C99/APP-cholesterol complexes in the bulk (non-raft) membranes can be cleaved by α-secretase is unclear. It is known that both full length APP and C99 can serve as substrates for α-secretase cleavage in the bulk membrane(138).