Abstract
Our group previously demonstrated a deficiency of migrating motor complexes in irritable bowel syndrome (IBS) patients with small intestinal bacterial overgrowth (SIBO). Based on disturbed fasting motility, we tested whether low-dose nocturnal erythromycin or tegaserod can prevent the recurrence of IBS symptoms after successful antibiotic treatment. Methods: 203 patient charts were reviewed to find IBS patients with SIBO, and treatment cycles were assessed to identify subjects with clinical and breath test resolution. The charts of those who met the inclusion criteria were reviewed to determine the method of prevention of symptom recurrence and the length of remission. The two preventive agents used were erythromycin (50 mg) or tegaserod (2–6 mg) orally at bedtime. Results: 64 patients met the inclusion criteria. Subjects receiving no prevention (n=6) after successful antibiotic treatment experienced symptom recurrence after 59.7±47.4 days. Prevention using erythromycin (n=42) demonstrated 138.5±132.2 symptom-free days (P=.08 vs no prevention) compared to 241.6±162.2 days with tegaserod (n=16; P=.003 vs no prevention; P=.004 vs erythromycin). Switching from erythromycin to tegaserod (n=20) extended resolution from 105.8±73.3 days to 199.7±162.9 days (P=.04). Changing from no therapy to erythromycin or tegaserod (n=6) extended recurrence from 41.0±44.8 days to 195.6±153.5 days (P=.06). Conclusion: Tegaserod significantly prevents the recurrence of IBS symptoms after antibiotic treatment compared to erythromycin or no prevention.
Keywords: Irritable bowel syndrome, migrating motor complexes, small intestinal bacterial overgrowth, erythromycin, tegaserod
Irritable bowel syndrome (IBS) is a common chronic condition affecting the gastrointestinal tract in up to 15% of the US population.1,2 Over the years, treatment has been symptom-based, but, recently, a new approach has been studied based on the hypothesis that IBS is caused by an abnormal distribution or quantity of gut microflora.3 This hypothesis suggests that patients with IBS may suffer from bacterial overgrowth based on indirect measurements such as breath testing. More recent evidence even suggests increased colony counts of bacteria in the small bowel in patients with IBS.4 This hypothesis has now become one of a growing number of hypothesis-based approaches to IBS.
To address this new hypothesis, three double-blind studies have been conducted and have demonstrated a benefit of antibiotic therapy in IBS.5-7 In a recent study, a 10-day course of rifaximin (Xifaxan, Salix) resulted in a benefit that lasted for the entire 10-week follow-up period of the study.7 The long-lasting duration of the benefit suggested that antibiotic therapy in IBS somehow corrected a causative factor. Although this was an optimistic development, bacterial overgrowth is known to be a recurring problem.
If bacterial overgrowth is a contributing factor in IBS symptoms, there must be a reason for this bacterial excess. Recent studies suggest that small bowel motor dysfunction may be a possibility. Specifically, a recent study suggested that the frequency of migrating motor complexes (MMCs) was lower among IBS patients with an abnormal lactulose breath test.8 In 1977, bacterial overgrowth was found to be associated with a lower frequency of MMC.9 Intuitively, it appears possible that this lack of MMC could lead to the bacterial overgrowth in IBS. If this is true, addressing this motility issue would be important in order to prevent bacterial overgrowth from recurring.
Tegaserod (Zelnorm, Novartis) is a 5-HT4 receptor agonist that has known accelerating effects on the small bowel as well as colon transit.10,11 On this basis, as well as due to its effectiveness in improving constipation clinically, tegaserod has been used to treat both constipation-pre-dominant IBS (C-IBS)12,13 and chronic constipation.14,15 However, tegaserod also has motor effects on the small intestine and specifically has been shown to influence fasting motility.16 Likewise, erythromycin, the motilin agonist, is known to increase the frequency of phase III interdigestive motility.17
In this study, we retrospectively evaluate the ability of tegaserod and erythromycin to prevent the recurrence of bacterial overgrowth and symptoms in IBS patients who were successfully treated with antibiotic therapy.
Methods
Patient Selection
A retrospective chart review was conducted on consecutive patients who were seen for consultation through the Gastrointestinal Motility Program at Cedars-Sinai Medical Center in Los Angeles, California. Inclusion into the study merited a two-step progression: a diagnosis of IBS that was treated on the basis of bacterial overgrowth origin and at least 1 successful treatment cycle for presumed small intestinal bacterial overgrowth (SIBO). Successful treatment was defined as clinical resolution of IBS symptoms. Patients were excluded for the following reasons: if, during the review, they were found to have inflammatory bowel disease, active peptic ulcer disease, documented microscopic colitis, or active gallbladder disease in addition to IBS; if they did not have a lactulose breath test to suggest bacterial overgrowth; if they did not achieve complete eradication of bacterial overgrowth after antibiotic treatment for their positive lactulose breath test; or if they had inadequate or no follow-up to accurately track at least 1 cycle of successful treatment and recurrence of bacterial overgrowth. The study was approved by the Cedars-Sinai Medical Center Institutional Review Board.
Review Process
Two hundred and three consecutive patient charts were identified and given a unique code to protect patient identity. After identifying patients with an explicitly stated IBS diagnosis, lactulose breath test results were checked for confirmation of bacterial overgrowth origin. Past medical history and medications were then assessed to determine treatment for overgrowth and symptoms (Figure 1). In order to provide a complete and accurate timeline of a patient’s management cycle of symptoms, treatments, and symptom recurrence, all progress notes, consultations, history, physicals, diagnostic tests, and breath tests were reviewed for all initial and subsequent visits. Instances of diarrhea, constipation, abdominal pain, bloating, excess gas, and stool frequency were recorded as well as the specific type of IBS: diarrhea-predominant IBS (D-IBS), C-IBS, or alternating IBS (A-IBS).
Figure 1.
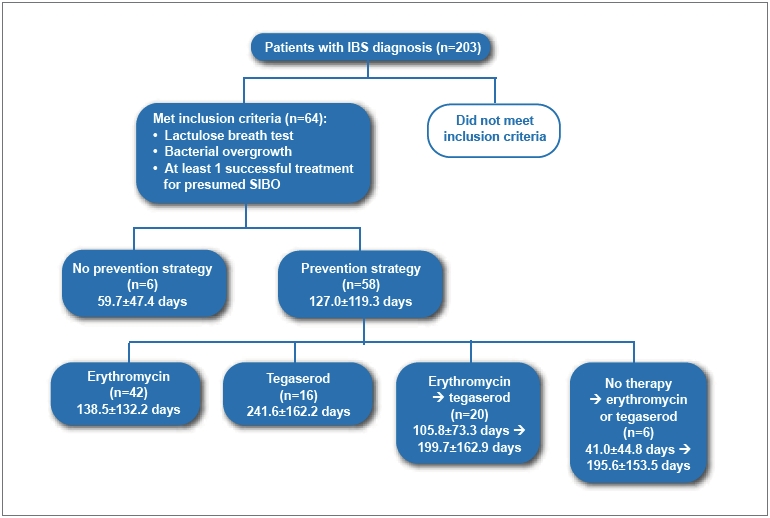
Review process for charts.
- IBS=
- irritable bowel syndrome
- SIBO=
- small intestinal bacterial overgrowth.
Most patients with positive lactulose breath tests were prescribed antibiotic treatment for 10 days or Vivonex™ elemental feeding formula (Nestle Nutrition) for 14 days as described in a previous study.18 Most patients returned to the center within 7 days of finishing their bacterial overgrowth acute treatment, and the efficacy of the treatment of their bacterial overgrowth was determined by a repeat lactulose breath test and relief from symptoms. The presence of an abnormal follow-up lactulose breath test or the reporting of a lack of symptom improvement denoted no eradication. Persistence of overgrowth usually resulted in a change of antibiotics or 2 weeks of Vivonex until eradication and symptom relief were accomplished.
Among those who achieved eradication and symptom improvement, most were given some form of preventive therapy. However, some subjects elected not to use preventive therapy (these were denoted as “no prevention”). Those who did receive a preventive treatment were given either erythromycin (50 mg via pediatric suspension; EES 200, Abbott) or low-dose tegaserod (2–6 mg) at bedtime daily. Investigators noted the type of preventive therapy used, the dosage, and the length of symptom relief (which was determined from the date of documented eradication until the date of reported symptom return and confirmed by a repeated lactulose breath test and patient symptom reporting).
Other Collected Information
Collected patient demographics included the age and sex of each patient as well as the type of preventive therapy used. If there was a relapse in symptom improvement, some patients were re-treated with antibiotics or Vivonex. If symptom resolution was achieved again, the preventive treatment was noted again. In many cases, the preventive strategy changed. For example, say a patient successfully achieved eradication of symptoms with an antibiotic but then relapsed when placed on erythromycin. For the second treatment, they may have been placed on tegaserod instead. All treatment cycles of a patient were recorded in this fashion.
Data Analysis
The chart review focused on the presence of bacterial overgrowth, success of antibiotic treatment, and length of symptom relief. As such, the investigators compared subjects who did not use any preventive therapy to subjects who used preventive therapy (erythromycin or tegaserod). Furthermore, in order to establish whether there was a difference in preventive treatments, a comparison of the success of remission maintenance was performed between erythromycin and tegaserod as well as a secondary comparison of the remission intervals for patients who first received erythromycin and then were switched to tegaserod on successive treatment cycles. Any differences in the success of prevention among patients with C-IBS, A-IBS, or D-IBS were compared.
Statistical Analysis
Qualitative demographic information was compared using Fisher’s Exact Test. The number of days to recurrence was compared among patients with no prevention, erythromycin, or tegaserod using a Wilcoxon rank sum test. To compare patients who changed from one therapy to another, a signed-ranks test was used for matched comparison.
Results
Patient Demographics
A total of 203 subjects were screened, of whom 64 met the inclusion criteria. Most subjects were excluded due to a lack of extensive follow-up (not enough data) or failure to achieve clinical remission during the documented time of clinical evaluation and treatment. This meant that the subjects had a successful clinical resolution of their IBS after antibiotics or Vivonex. The type and distribution of this therapy is summarized in Table 1. Of these patients, 41 (64%) were women. The average age of the study cohort was 48.7±16.1 years. Six patients constituted the no-prevention arm, and the remaining 58 patients received some form of preventive maintenance (42 received erythromycin, and 16 received tegaserod). The demographics of these three groups were similar (Table 2). There were 20 patients who began on erythromycin and were later switched to tegaserod. The average number of antibiotic or Vivonex treatment cycles per patient was 1.7 cycles.
Table 1.
Summary of Subjects Included in the Study
| Antibiotic or supplement | Number of subjects |
|---|---|
| Ampicillin/clavulanate | 8 |
| Ampicillin/clavulanate + doxycycline | 1 |
| Ciprofloxacin | 4 |
| Doxycycline | 4 |
| Neomycin | 6 |
| Norfloxacin | 1 |
| Rifaximin | 17 |
| Rifaximin + neomycin | 2 |
| Vivonex elemental feeding formula | 21 |
| Total | 64 |
Table 2.
Demographics of Subjects Based Upon Type of Prevention Strategy Received
| Prevention strategy | |||
|---|---|---|---|
| No difference was seen among the groups. | |||
| Demographics | None (n=6) | Erythromycin (n=42) | Tegaserod (n=16) |
| Age (years) | 42.8±21.3 | 50.7±15.8 | 45.6±15.1 |
| Sex (male/female) | 2/4 | 17/25 | 4/12 |
Prevention Therapy With First Treatment
After the first successful antibiotic treatment, symptoms recurred an average of 59.7±47.5 days later when no prevention therapy was used (n=6). This was compared to 138.5±132.2 days (P=.08 vs no prevention) if erythromycin was used for prevention and 241.6±162.2 days (P=.003 vs no prevention; P=.004 vs erythromycin) if tegaserod was used (Figure 2).
Figure 2.
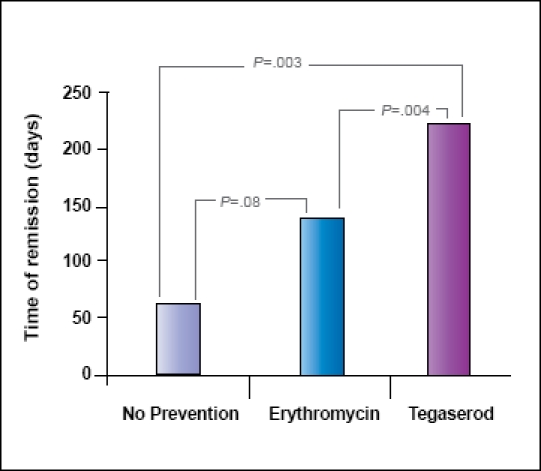
Duration of clinical and breath test remission with the three posteradication prevention measures.
All Prevention Therapies
Many patients needed additional antibiotic treatment cycles after symptom recurrence on the preventive strategy they were using. Thus, some patients used more than one type of preventive maintenance. In this analysis, all preventive strategies (including re-treated patients) were collected. Again, the groups were no-prevention strategy (n=9), erythromycin (n=56), and tegaserod (n=33). For patients using prevention, the average recurrence of sym ptoms occurred 50.4±40.4 days after eradication. The average recurrence was 146.0±131.5 days (P=.005 vs no prevention) after eradication in patients using erythromycin and 216.0±160.7 days (P=.003 vs no prevention and P=.008 vs erythromycin) after eradication in patients using tegaserod (Figure 3).
Figure 3.
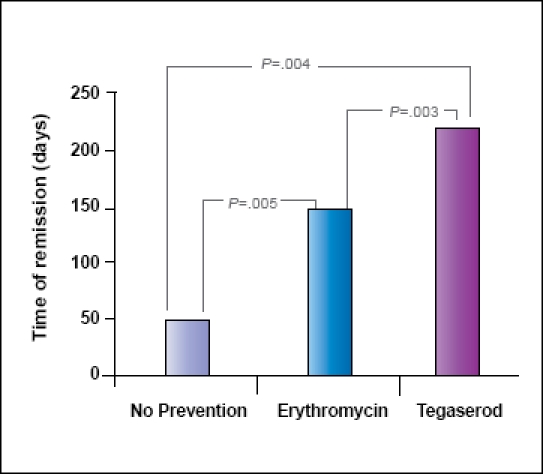
Any successful treatment with follow-up prevention or no prevention. No prevention (n=9), erythromycin (n=56), and tegaserod (n=33).
Switching From Erythromycin to Tegaserod
Five patients with no therapy who were later given erythromycin or tegaserod extended prevention of symptom recurrence from 41.0±44.8 days to 195.6±153.5 days (P=.06; Figure 4A). In 20 patients who experienced symptom recurrence with erythromycin, tegaserod was used after another antibiotic therapy. The number of days of prevention extended from 105.8±73.3 days with erythromycin to 199.7±162.9 days for tegaserod (P=.04; Figure 4B). In addition, 2 patients stopped taking tegaserod after 209 and 365 days of normal bowel function. After stopping tegaserod, symptoms recurred in 30 and 45 days, respectively.
Figure 4.
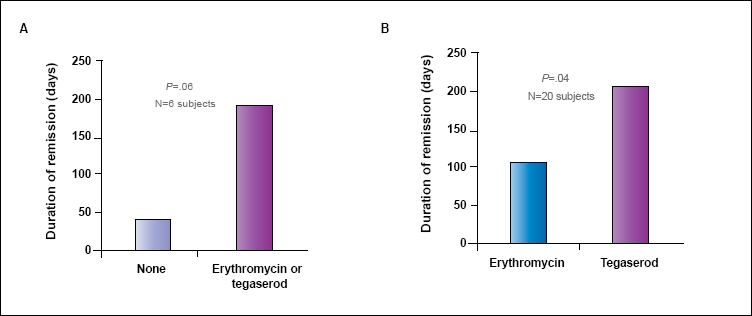
Symptom relapse among subjects who initially fail with no prevention and proceed to erythromycin or tegaserod (A). Symptom relapse among subjects who relapse on erythromycin as a prevention strategy and subsequently switch to tegaserod (B).
Constipation-Predominant Irritable Bowel Syndrome Versus Diarrhea-Predominant Irritable Bowel Syndrome
Among all subjects taking tegaserod at some point, 14 had C-IBS. These C-IBS subjects were symptom-free using nocturnal tegaserod after antibiotics or Vivonex for 192.8±128.4 days. Ten D-IBS patients who used tegaserod were symptom-free for 287.7±236.3 days (P=.13 vs C-IBS). Seven subjects had both diarrhea and constipation IBS (A-IBS). The time to recurrence in this group was 212.7±99.9 days (no difference from C-IBS or D-IBS; Figure 5).
Figure 5.
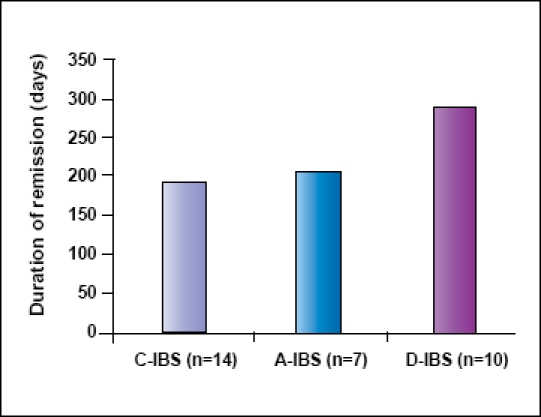
The ability of tegaserod to prevent relapse based upon the type of irritable bowel syndrome at the initial visit.
- A-IBS=
- alternating-type irritable bowel syndrome
- C-IBS=
- constipation-predominant irritable bowel syndrome
- D-IBS=
- diarrhea-predominant irritable bowel syndrome
There was no statistical significance among the groups.
Discussion
In this study, we demonstrate for the first time that prokinetic therapy appears to maintain remission of symptom recurrence in IBS patients after treatments based on a bacterial overgrowth hypothesis. Nocturnal administration of both erythromycin and tegaserod demonstrate significant prevention of IBS symptom recurrence. However, a greater benefit is seen with tegaserod compared to erythromycin. Interestingly, the duration of prevention is greater in D-IBS than in C-IBS, though the difference is not statistically significant (Figure 5).
The concept of treating IBS patients with antibiotics stems from data demonstrating that IBS patients have an increased prevalence of bacterial overgrowth, which is often measured by lactulose breath tests. Results of recent studies indicate that up to 84% of patients with IBS have an abnormal lactulose breath test5 suggestive of SIBO. Although there is some criticism regarding the accuracy of lactulose breath tests in diagnosing bacterial overgrowth, recent data support a role for gut bacteria on two fronts. A recently published paper by Posserud and associates found that, after hours of water perfusion manometry followed by aspiration and culture (which would dilute colony counts), IBS patients did not often have more than 105 bacteria in the small bowel; however, the paper did find an overall increase in the number of colony counts in the small bowel in IBS patients compared to healthy control subjects.4 The second important finding that dictates the consideration of gut bacteria in the manifestations of IBS is the response to antibiotic therapy. Three double-blind studies have now suggested that IBS subjects improve significantly after neomycin or rifaximin therapy.5-7
Two difficulties arise with the assumption that bacterial overgrowth is a potential cause of IBS. Historically, there has been a cause for bacterial overgrowth and, thus, the state of excess bacteria recurs. This leads to the potential need for repetitive courses of antibiotics. Neomycin appears to produce clinical resistance in this framework of treating IBS.19 Fortunately, no such resistance is seen with rifaximin.19 However, as with other bacterial overgrowth, the best method of management is treatment of the underlying cause for build-up.
In the absence of any structural cause of bacterial overgrowth, Vantrappen and colleagues demonstrated a reduction in the frequency of phase III interdigestive motility.9 This reduction in phase III was a presumed mechanism leading to bacterial overgrowth recurrence. Phase III interdigestive motility was first described in 1969 by Szurszewski and is believed to be responsible for cleansing the small bowel of residual debris from meals.20 Since the time of these studies, inhibition of fasting motor function and phase III activity using narcotics has been found to result in bacterial overgrowth as well.21,22
Based on recent study, the presentation of bacterial overgrowth in IBS could also similarly be related to a reduction in this fasting motor pattern. In a recent study, IBS subjects with a positive lactulose breath test, suggesting bacterial overgrowth, had a significantly reduced frequency of phase III compared to healthy controls.8 Although not thought to be as common as in this IBS study,8 other studies have found similar results in IBS patients with proven bacterial overgrowth.4 If a reduction in phase III is a potential contributor to symptom-producing bacterial overgrowth, therapy directed at this motor activity may result in the maintenance of the clinical resolution of bacterial overgrowth in IBS after initial antibiotic treatment.
Two prokinetics have the potential to stimulate motor events during fasting motility. The first is erythromycin. Although its primary use is as an antibiotic, erythromycin, at low doses, has shown kinetic effects on fasting motility, specifically for phase III.17 This is seen at doses as low as 50 mg. The second potential drug that could induce fasting motility is tegaserod. Although not specifically approved for this indication, studies do suggest that it may have activity on fasting small bowel motor function as well as known postprandial effects on colon motor activity leading to bowel movements.16 Tegaserod was approved for C-IBS because of this latter effect.
In this study, we show that after successful treatment of IBS on the basis of bacterial overgrowth, symptom relief of IBS is greater with the use of preventive therapy. Although both erythromycin and tegaserod delay the recurrence of symptoms, it appears that tegaserod is superior to erythromycin (Figure 2). Remission is four times longer with tegaserod than with no therapy and nearly twice as long as erythromycin. Although only 2 patients discontinued tegaserod in this review, their time to symptom recurrence was similar to that with no prevention, providing further evidence of the effectiveness of tegaserod. In addition, those patients noted to relapse on erythromycin only to switch to tegaserod after another induction with SIBO treatment stayed in remission longer. This suggests that the failure of erythromycin does not predict a group of patients more refractory to a prokinetic effect of prevention, as the number of days in remission in Figure 4B was similar to that of people who only used tegaserod in Figure 2.
One issue of concern is the use of tegaserod in patients with D-IBS. Tegaserod was initially approved for the treatment of C-IBS12,13 and later chronic constipation.14,15 There was initial concern over the use of tegaserod in patients with D-IBS, as a known side effect of tegaserod can be diarrhea. In this study, we observed that, although not statistically significant, the prevention of recurrence is more pronounced in patients with D-IBS. There are two possible explanations for the lack of diarrheal side effects with tegaserod in D-IBS. The first is that the diarrhea was already treated with the course of antibiotic or Vivonex. Thus, patients no longer had the worrisome symptom that tegaserod could potentiate. The second reason may be the use of tegaserod. The primary effect of tegaserod is on postprandial colonic motor activity. It likely potentiates the motor events activated at the time of drug administration. In the fasting state at bedtime, these events are not present and the only inducible event would be small bowel motor events such as phase III. Nevertheless, diarrhea was not seen as a significant effect of tegaserod in the D-IBS group in our study, and this group fared better than even C-IBS.
One difficulty with applying the study results above to clinical practice is the lack of availability of tegaserod. In 2007, tegaserod was withdrawn from the US market due to concerns regarding rare cerebrovascular events noted in clinical trials. Thus, it is not possible to use tegaserod in this setting. However, erythromycin is currently available.
Although the data appear very significant, there are some recognized limitations in this study. First, the number of subjects who received no preventive therapy is small. This is due to the fact that in 2005, our technique was to uniformly place all patients on preventive maintenance. We found some patients without maintenance therapy because some of them were seen in 2005 but had not been in the clinic since 1999, before tegaserod was introduced. We chose the year 2005 specifically for this chart review because it would allow us to observe patients who were likely to have straddled a period of time where some of them may have charted back to 1999 (for the evaluation of no treatment) and some would have received erythromycin, and also because 2005 was long enough after the release of tegaserod in the US market to evaluate long-term efficacy of the drug. Another major limitation of this study is its uncontrolled, retrospective design. The purpose of this study was to evaluate the concept of nonantibiotic prevention in the management of SIBO. These data were to provide a guide for powering a large-scale randomized study for the prevention of SIBO. Unfortunately, the removal of tegaserod from the US market in 2007 prevented this next undertaking. However, the overuse of antibiotics is such an important issue that these data should be available to the prescribing community if they can reduce the need for repetitive antibiotics.
Overall, as more data accumulate on the benefit of antibiotics in the treatment of IBS, techniques are needed to avoid the traditional approach of using rotating courses of antibiotics. This study represents a first step in that direction. Tegaserod appears to be an ideal therapy for maintaining remission of symptoms in all IBS subtypes. Future work should include controlled trials using prokinetics in development as a means of preventing recurrence of symptoms in IBS patients treated with antibiotics.
Acknowledgments
This study was supported by an investigator-initiated grant from Novartis Pharmaceuticals.
We would like to acknowledge the Beatrice and Samuel A. Seaver Foundation for their continued support of our efforts to study the pathophysiology of IBS.
References
- 1.Drossman DA, Sandler RS, McKee DC, Lovitz AJ. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology. 1982;83:529–534. [PubMed] [Google Scholar]
- 2.Thompson WG, Heaton KW. Functional bowel disorders in apparently healthy people. Gastroenterology. 1980;79:283–288. [PubMed] [Google Scholar]
- 3.Lee HR, Pimentel M. Bacteria and irritable bowel syndrome: the evidence for small intestinal bacterial overgrowth. Curr Gastroenterol Rep. 2006;8:305–311. doi: 10.1007/s11894-006-0051-3. [DOI] [PubMed] [Google Scholar]
- 4.Posserud I, Stotzer PO. Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 6.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 7.Pimentel M, Park S, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 8.Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47:2639–2643. doi: 10.1023/a:1021039032413. [DOI] [PubMed] [Google Scholar]
- 9.Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158–1166. doi: 10.1172/JCI108740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 11.Degen L, Petrig C, Studer D, Schroller S, Beglinger C. Effect of tegaserod on gut transit in male and female subjects. Neurogastroenterol Motil. 2005;17:821–826. doi: 10.1111/j.1365-2982.2005.00715.x. [DOI] [PubMed] [Google Scholar]
- 12.Müller-Lissner SA, Fumagalli I, Bardhan KD, Pace F, Pecher E, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655–1666. doi: 10.1046/j.1365-2036.2001.01094.x. [DOI] [PubMed] [Google Scholar]
- 13.Novick J, Miner P, Krause R, Glebas K, Bliesath H, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16:1877–1888. doi: 10.1046/j.1365-2036.2002.01372.x. [DOI] [PubMed] [Google Scholar]
- 14.Kamm MA, Müller-Lissner S, Talley NJ, Tack J, Boeckxstaens G, et al. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005;100:362–372. doi: 10.1111/j.1572-0241.2005.40749.x. [DOI] [PubMed] [Google Scholar]
- 15.Johanson JF, Wald A, Tougas G, Chey WD, Novick JS, et al. Effect of tegaserod in chronic constipation: a randomized, double-blind, controlled trial. Clin Gastroenterol Hepatol. 2004;2:796–805. doi: 10.1016/s1542-3565(04)00356-8. [DOI] [PubMed] [Google Scholar]
- 16.DiStefano M, Vos R, Janssens J, Tack JF. Effect of tegaserod, a 5HT4 receptor partial agonist, on interdigestive and post-prandial gastrointestinal motility in healthy volunteers. Gastroenterology. 2003;124:A163. [Google Scholar]
- 17.Otterson MF, Sarna SK. Gastrointestinal motor effects of erythromycin. Am J Physiol. 1990;259(3 pt 1):G355–G363. doi: 10.1152/ajpgi.1990.259.3.G355. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel M, Constantino T, Kong Y, Bajwa M, Rezaei A, Park S. A 14-day elemental diet is highly effective in normalizing the lactulose breath test. Dig Dis Sci. 2004;49:73–77. doi: 10.1023/b:ddas.0000011605.43979.e1. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Lee HR, Low K, Chatterjee S, Pimentel M. Rifaximin versus other antibiotics in the primary treatment and re-treatment of bacterial overgrowth in IBS. Dig Dis Sci. 2008;53:169–174. doi: 10.1007/s10620-007-9839-8. [DOI] [PubMed] [Google Scholar]
- 20.Szurszewski JH. A migrating electric complex of canine small intestine. Am J Physiol. 1969;217:1757–1763. doi: 10.1152/ajplegacy.1969.217.6.1757. [DOI] [PubMed] [Google Scholar]
- 21.Kuperman DA, Sninsky CA, Lynch DE. Myoelectric activity of the small intestine during morphine dependence and withdrawal in rats. Am J Physiol. 1987;252:G562–G567. doi: 10.1152/ajpgi.1987.252.4.G562. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwenhuijs VB, Verheem A, van Duijvenbode-Beumer H, Visser MR, Verhoef J, et al. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg. 1998;228:188–193. doi: 10.1097/00000658-199808000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


