Summary
The development of volume replacement fluids for resuscitation in hemorrhagic shock comprises oxygen carrying and non carrying fluids. Non oxygen carrying fluids or plasma expanders are used up to the transfusion trigger, and upon reaching this landmark either blood, and possibly in the near future oxygen carrying blood substitutes, are used. An experimental program in hemorrhagic shock using the hamster chamber window model allowed to compare the relative performance of most fluids proposed for shock resuscitation. This model allows investigating simultaneously the microcirculation and systemic reactions, in the awake condition, in a tissue isolated from the environment. Results from this program show that in general plasma expanders such as Ringer’s lactate and dextran 70 kDa do not sufficiently restore blood viscosity upon reaching the transfusion trigger, causing microvascular collapse. This is in part restored by a blood transfusion, independently of the oxygen carrying capacity of red blood cells. These results lead to the proposal that effective blood substitutes must be designed to prevent microvascular collapse, manifested in the decrease of functional capillary density. Achievement of this goal, in combination with the increase of oxygen affinity, significantly postpones the need for a blood transfusion, and lowers the total requirement of restoration of intrinsic oxygen carrying capacity.
Keywords: Plasma expander, blood viscosity, oxygen carrying capacity, blood substitutes, hemorrhagic shock, microcirculation
Introduction
The transfusion trigger is usually defined by an array of physiological parameters which in combination with decreased intrinsic oxygen carrying capacity of blood (circulating hemoglobin concentration, or hematocrit, Hct) determines the decision to switch from volume maintenance by means of plasma expanders to the transfusion red blood cells (RBCs). It is virtually universally perceived that the requirement for blood is determined by the impending risk of anoxia, particularly in the vital organs, and it is general accepted that the transfusion of RBCs improves patients’ conditions.
Whether the beneficial effect of a blood transfusion is due to the restoration of oxygen carrying capacity is debatable, because RBCs in general deliver little oxygen upon transfusion, particularly if they have been stored for some time (more than a few days)[1]. The almost universal practice of transfusing the oldest RBCs in storage insures that in most instances there is a comparatively reduced initial restoration of oxygen carrying capacity, since full oxygen delivery is re-established several hours after a blood transfusion.
Blood transfusion are usually called for when the circulating blood hemoglobin reaches the level of 7 g/dl, this value being lower in young, normal individuals, and higher for elder persons. When blood reaches this hemoglobin concentration, Hct and all blood factors have fallen to about half of the normal value. A parameter that may decrease to a greater extent is blood viscosity, due to its approximately exponential dependence on Hct [2]. Notably blood viscosity is a parameter that directly links systemic blood pressure and cardiac output. It is a well established fact that at hemodilution levels of about 50% of normal Hct there is no fall in either blood pressure or cardiac output if normovolemia is maintained [3]. An explanation for this result is that this level of hemodilution elicits an increase of cardiac output and some level vasoconstriction, probably due to the combination of baroreceptor reflexes, and the decrease of shear stress on the vascular wall, which lowers nitric oxide (NO) production promoting vasoconstriction and the increase of peripheral vascular resistance [4].
Microvascular function in vasoconstriction
In clinical settings related to different hipotensive shock conditions vasoconstriction is instituted to insure the perfusion of central organs. As an example, vasopressin is prescribed for increasing blood pressure when the organism does not respond to other interventions [5]. A hidden component of this process is that vasoconstriction mostly occurs at the level of arterioles, whereby upstream blood pressure is increased, while pressure in the capillary compartment is lowered [6]. While in principle this accomplishes the objective of elevating the pressure necessary for perfusion, there is evidence that increasing blood pressure by causing vasoconstriction has potentially negative consequences since it lowers functional capillary density (FCD).
A critical element of this process is the evolution of FCD, a parameter used in microvascular physiology to describe microscopic tissue perfusion. It quantifies capillary perfusion by measuring the total length of capillaries that have transit of RBCs in a period of 30 s per unit area of tissue observed by intravital microscopy. The significance of FCD was first proposed by Fagrell, 1977 [7], who established its relevance in tissue survival. In hemorrhagic shock survival is determined by maintenance of FCD and is mostly independent of tissue oxygenation [8].
Attempting to re-establish perfusion of the vital organs by vasoconstriction to elevate blood pressure has a negative impact on capillary perfusion because FCD is a function of local capillary pressure, which lowers downstream of the constricted arterioles. Maintenance of adequate levels of FCD is usually associated with an adequate supply of oxygen to the tissue. Additionally functional capillaries are also necessary for the extraction of the products of metabolism from the tissue which otherwise accumulate. This is evidenced in extreme hemodilution, a process that affects tissue oxygenation independently of the changes of FCD, but presents a significant difference in outcome in terms of acid base balance depending on whether the plasma expander used maintains FCD above a minimum threshold [9] [6].
What is adequate oxygen delivery?
This question does not have a simple answer because there is no oxygen related parameter that unequivocally characterizes the need to restore or maintain oxygen delivery capacity in the organism, with the exception of obviously extreme cases. In general there is the tendency of over emphasizing oxygen delivery, and upon reaching either measured or perceived borderline conditions blood transfusions are prescribed, overlooking the fact that one in 10 transfusions present complications, and one in 100 cause severe problems. Tissue pO2 is often taken as a marker for blood transfusion, however this is a moving target since it is only partially related to oxygen delivery and consumption, and it is not possible to assess in the vital organs.
Conventional tissue oxymetry measures average blood saturation in the tissue, combining arteriolar and venular blood. The pO2 of mixed venous blood is distantly related to tissue pO2, and tissue whose metabolic function is severely compromised uses little oxygen and can have a high pO2, since moribund tissue does not consume oxygen as shown by Cabrales et al., 2006,[10] who found that inhibiting tissue metabolism increases tissue pO2. This study showed that performing a 80% exchange transfusion of molecular hemoglobin caused the reduction of microvascular oxygen delivery and extraction in the tissue of the hamster window chamber and the elevation of interstitial pO2 above normal levels.
From a physiological viewpoint there are two components that define adequate tissue oxygenation. One is that the tissue must receive oxygen at a rate sufficient to sustain its metabolic requirements. Secondly and directly linked with the need to sustain metabolism, oxygen delivery must also insure that the tissue is uniformly supplied with oxygen, in such a fashion that there are no anoxic foci, a condition also termed focal ischemia. In this context average tissue pO2 at rest is usually found to be in the range of 20 to 25 mmHg. A recent study in the hamster window chamber window model, where measurements can be made without anesthesia, and isolated from the environment showed that tissue pO2 had a Gaussian distribution averaging 20.2 ± 7.7 mmHg, range 4.8 – 35.5 mmHg. In this study pO2 was measured in areas 20 × 20 μm using the phosphorescence quenching [11] technique [12] in regions of the tissue void of microvessels. The absolute value of the standard deviation is significant because it shows what percentage of the tissue may be at risk of being anoxic. In the Gaussian distribution, two standard deviations away from the mean represent 2.5% of the total tissue, or the probability of finding a microscopic region at the risk of anoxia. It should be noted that this variability comprises both the intrinsic biological variability and that arising from the methodology per se. Furthermore there is a natural temporal variability arising from the continuous local adjustment of microvascular flow due to vasomotion [13].
The NAD/NADH (NAD: Nicotinamide adenine dinucleotide) redox state is a sensitive indicator of intracellular oxygen tension [14]. NADH fluorescence is a specific marker of tissue hypoxia and can be determined precisely by optical methods [15]. The decrease of oxygen concentration decreases NAD and other components of the mitochondrial respiratory chain which become reduced [16, 17]. The critical interstitial and intravascular pO2 associated with the switch to anaerobic metabolism are reported to be below 2 and 7 mmHg respectively, indicating that oxygen delivery is regulated at levels well above the minimum required for oxidative metabolism. The oxygen tension required at the mitochondrial level to support oxidative metabolism (critical pO2) is <1 mmHg, whereas tissue oxygen tension reported in tissues in vivo is in the range 15 – 25 mmHg [15].
These considerations suggest that adequate oxygen delivery should fulfill both a quantitative and qualitative criteria; where for the second requirement the presence of near normal FCD plays a critical role.
Resuscitation from hemorrhagic shock
Hemorrhagic shock, even in standardized animal experiments, is a composite pathophysiological condition, where local and central controls interact. Regardless of this inherent complexity, it provides a fairly definitive means for understanding the interplay between perfusion and intrinsic blood oxygen carrying capacity in sustaining the tissue. A conventional shock experiment consists in withdrawing 50% of an animal’s blood volume, waiting one hour, and returning the animal to normovolemia by infusing 25% of the shed volume, using either blood or a plasma expander (the remaining 25% is contributed by autotransfusion from the tissue fluid). A variety of oxygen carrying and non carrying volume restoration fluids can be used experimentally to evaluate the merit of restoring perfusion vs. intrinsic oxygen carrying capacity including conventional plasma expanders, molecular hemoglobin based solutions, vesicle encapsulated oxygen carriers, and the transfusion of blood with modified RBCs.
A meaningful comparison in terms of outcome in considering the different biophysical properties of volume/oxygen carrying fluids could be made by analyzing the outcome of the basic resuscitation fluids that have been studied in the setting of the chamber window hamster preparation. Clearly this model is far removed from the realities of shock resuscitation in humans, however it is a standard model that has allowed investigating both systemic and microvascular parameters in the awake condition, in a tissue that is isolated from the environment, and therefore not subjected to unknown reactions due to exposure. This comparison avoids the need of justifying differences between species, methodologies and laboratory settings.
There is presently a significant background of information on the responses of the hamster chamber window model to a resuscitation following hipovolemic hemorrhagic shock. In the following we will make some basic comparisons by relating the type of resuscitation fluid, its oxygen carrying capacity, and its capacity to restore blood viscosity to near normal levels. The conventional fluids that serve as baseline are Ringer’s lactate, blood, 70 kDa dextran and hydroxyethyl starch (HES). These fluids will be compared with results obtained with molecular hemoglobin solutions, polyethylene glycol modified albumin and hemoglobin (PEG-Alb & PEG-Hb respectively), hemoglobin vesicles and non oxygen carrying RBCs.
Findings with blood, oxygen carrying and non carrying plasma expanders
Basic resuscitation fluids: Ringer’s lactate, dextran and blood
The study of Kerger et al., 1997, provided the basic comparative data for this micro/macro model [18]. This study used a 2 hour shock model. Resuscitation was implemented with 50% volume restoration for shed blood and dextran, and 100% volume restoration for Ringer’s lactate. In this study blood pressure was immediately restored by blood, with full restoration at 24 hours with all fluids. In general blood provided a significantly improved initial recovery of all parameters, particularly blood pressure and FCD. Notably both dextran 70 kDa and Ringer’s lactate caused persistent microvascular flow impairment and severe tissue hypoxia.
Molecular hemoglobin, crosslinked and oligomerized human hemoglobin (Hemolink)
This material produced by Hemosol Inc., Toronto, Canada, was developed following the trend prevalent until recently that assumed that blood substitutes should restore most of the oxygen carrying capacity of shed blood, that the mixture of remaining blood and resuscitation fluid having a viscosity lower than natural blood is beneficial according to the experience with hemodilution, that oxygen affinity should be low to facilitate oxygen release, and that the pervasive hypertensive response to this type of products was beneficial since it facilitated the recovery of blood pressure. Products with these characteristics have to the present not been succesful in being accepted as blood substitutes since molecular hemoglobin based vasoconstriction appears to cause myocardial lesions in primates as found with the use of αα-hemoglobin (HemeAssist, intramolecular cross linked with diaspirin, Baxter Healthcare Corp., Boulder, Co.) [19]. Other products presently in development like PolyHeme (glutaraldehyde cross linked pyridoxalated human hemoglobin, Northfield Laboratories Inc., Evanston, IL) and Hemopure (Biopure Corporation, Cambridge, MA), a low affinity bovine Hb polymerized with glutaraldehyde presently in clinical trials, both materials appearing to have similar physiological characteristics in terms of their vasoactivity.
Hemolink (o-raffinose polymerized human hemoglobin) was used in the two hour shock model [18]. Its concentration (10 gHb/dL) determined that blood hemoglobin restoration was similar to that attained with whole blood. Blood pressure recovery was significantly better using blood, while FCD recovery was significantly less as might expected from a material that causes vasoconstriction.
Glutaraldehyde polymerized bovine hemoglobin (PBH, Oxyglobin, a veterinary product, 13g Hb/dl, Biopure, Boston, MA) was used in same resuscitation protocol. Hemoglobin was significantly higher, 8.8 ± 0.3 g/dl, using PBH when compared to 5.7 ± 0.5 in HSA resuscitated animals (p < 0.05), resuscitation with PBH leading to a significant increase in tissue pO2 by comparison with HSA. However, increased tissue pO2 was not critical in restoring metabolic function since base excess and pH were restored to baseline levels with both PBH and HSA, even though a significant deficit in microcirculatory blood flow persisted in both groups [20].
Both results (Hemolink and Oxyglobin) should be the consequence of its vasoactivity, since vasoconstriction of the arterioles increases central blood pressure, but lowers downstream capillary pressure.
PEG-albumin vs. hydroxyethyl starch resuscitation
Resuscitation was implemented restoring blood volume with polyethylene glycol conjugated bovine serum albumin (12 copies of PEG 5 kDa, PEG-Alb, 2.5 g/dl) and hydroxyethyl starch (200 kDa HES, 10% w/vol in 0.9% saline, Pentaspan, B. Braun Medical, Irvine, CA) delivered as 50% of the shed volume. Resuscitation with PEG-Alb restored systemic and microvascular parameters up to the end of the observation period (90 min). HES was identical to PEG-Alb resuscitation during the initial 10 – 15 min but effects were not subsequently sustained. The trend of recovery of MAP for HES persisted beyond the time when both FCD and tissue pH decreased thus MAP was not indicative of early microvascular dysfunction. The early difference in outcome found at the microvascular level with these resuscitation solutions suggests that the period in which to make data based decisions for resuscitation may be short and on the order of minutes. In this experiment final plasma viscosity was 1.4 cP for PEG-Alb, and 1.1 cP for HES [21]; as a reference normal plasma viscosity is 1.2 ± 0.1 cP.
PEG-Albumin vs. PEG-hemoglbin
PEG-hemoglobin is an oxygen carrying plasma expander (MP4, Sangart Inc., San Diego, CA) presently in clinical trials.. In these shock experiments resuscitation was made using albumin and hemoglobin molecules conjugated with PEG using the same chemistry, and delivered at the same concentration (4.2 g/dl) [22]. Thus differences in outcome should be directly attributable to difference in oxygen transport capacity (and to a lesser degree to the differences in colloid osmotic pressure (37 mmHg for PEG-Alb vs. 49 mmHg PEG-Hb). Both compounds had viscosities in the range of 2.5 – 2.7 cP. As expected blood hemoglobin concentration after shock resuscitation was higher with PEG-Hb, being 7.5 ± 0.3 vs. 6.5 ± 0.4 g/dL. This difference in oxygen carrying capacity is small (15% increase), however oxygen delivery increase by 67% and oxygen extraction by 43%. This disproportionate increase in oxygen availability by comparison to the increase in oxygen carrying capacity should be primarily the consequence of the very low p50 of PEG-Hb (~ 5 mmHg) which results in this material unloading oxygen only in regions with a significant oxygen deficit. The presence of this oxygen deficit was evidenced by PEG-Alb resulting in a tissue pO2 (chamber window model) of 5 ± 2 mmHg, with 50% of the tissue at the risk of anoxia, vs. a tissue pO2 of 8 ± 3 mmHg for PEG-Hb, where the risk of anoxia was circumscribed to 16% of the tissue.
A conclusion from these studies is that in general blood is a better resuscitation fluid than conventional plasma expanders and small hemoglobin molecules based oxygen carrying plasma expanders. A notable departure from this assertion is the results obtained with PEG-Alb and PEG Hb. The non oxygen carrying PEG-Alb was found to be superior to all the remaining plasma expanders, while its oxygen carrying counterpart, PEG-Hb was found to be equal to blood in its microvascular and blood pressure recovery properties, although not in its oxygen delivery properties. The latter was clearly due to the small hemoglobin concentration of this compound, resulting in a comparatively small restoration of blood intrinsic oxygen carrying capacity.
The improved results of obtained with the use of PEG products appears to be directly related to the recovery of microvascular function and particularly FCD that is obtained upon application of these materials. A physiological mechanism behind these effects should be related to the increase plasma viscosity, which in studies of extreme hemodilution was found to maintain microvascular function when compared to similar but less viscous plasma expanders[9]. These considerations led to analyzing the shock resuscitation with different formulations of viscous plasma expanders.
Resuscitation with hyperviscous solutions
Analysis of the results discussed shows that a common denominator of the more effective resuscitation fluids whether oxygen carrying or no carrying is that they increase plasma viscosity. Increasing plasma viscosity is accomplished by either the introduction of large molecular species, or the increase in number of solute molecules, both approaches having biological limitations. Increasing the number of molecules increases the osmotic pressure, while increasing molecular dimensions, increase the tendency to promote coagulation disorders. The increase in osmotic pressure is self limiting, since this will bring more fluid into the circulation, with the results that the material is diluted and the desired viscosity increase is not attained. Thus to increase plasma viscosity it is necessary to increase the molecular dimension of the solute.
Hyperviscous & hyperoncotic HES solutions, hemorrhagic shock
Wettstein et al. studied the effects using increased concentrations of hydroxethyl starch (HES) in the hamster shock resuscitation protocol [23]. Awake hamsters were subjected to 50% hemorrhage and were resuscitated with 25% of the estimated blood volume with 5, 10 or 20% solution of HES (Pentaspan, B. Braun Medical Inc., Irvine, CA). The increase in concentration led to an increase in COP (from 20 to 70 and 194 mmHg) and viscosity (from 1.7 to 3.8 and 14.4 cP). Cardiac index, microcirculatory and metabolic recovery were improved with HES 10 and 20% when compared to 5% HES. Oxygen delivery and consumption in the dorsal skin fold chamber was more than doubled with HES 10% and 20% when compared to HES 5%. This was attributed to the beneficial effect of restored or increased plasma COP and plasma viscosity as obtained with HES 10% and 20% leading to improved microcirculatory blood flow values early in the resuscitation period. The increase in COP led to an increase in blood volume as shown by a reduction in hematocrit. Mean arterial pressure was significantly improved in animals receiving 10 and 20% solutions. In conclusion, these results showed that the increase in concentration of HES, leading to hyperoncotic and hyperviscous solutions is beneficial for resuscitation from hemorrhagic shock since normalization of COP and viscosity led to a rapid recovery of microcirculatory parameters [23].
Hyperviscous & hyperoncotic HES solutions, hemorrhagic shock & continuous bleeding
A variation of the previous protocol was the inclusion of continuous bleeding following resuscitation from hemorrhagic shock (50% of blood volume, BV) followed by bleeding at the rate of 20% of BV per hour, with blood losses equaling 100% of total BV [24]. A single volume infusion (resuscitation) was performed 50 minutes after hemorrhage using 25% of BV with 10% hydroxyethyl starch (HES 200, group HES4), or a mixture of HES 200 with 0.3 or 0.6 % wt/vol alginate (group HES7 and HES10, respectively) leading to solutions with a uniform colloidal oncotic pressure (84 to 87 mmHg) and viscosities ranging from 3.8 to 9.8 cp. Alginate (Novamatrix/FMCBiopolymer, Norway) are very high molecular materials with comparatively high viscosities at very low concentrations that have minimal (a few mmHg) oncotic activity. All solutions had similar initial (after resuscitation, 10 – 15 min) effects that diverged thereafter. The viscosity enhanced solutions showed improved and longer lasting (90 min) maintenance of arterial blood pressure, microvascular flow, capillary perfusion and laboratory parameters than the low viscosity solutions. Low (conventional (viscosity) resuscitation caused all microvascular parameters to return to the shock level 90 min after. Therefore this experimental investigation showed that viscosity per se is a leading cause of improved resuscitation since the extent of recovery in the presence of continuous bleeding was unaffected by differences in blood volume changes, given that all solutions had the same COP.
Hyperviscous-hyperoncotic small volume hemorrhagic shock resuscitation
In these experiments resuscitation was produced in the conventional 1 hr shock protocol by the introduction of 7.5 NaCl solution, 7% of shed blood volume, followed by the infusion of 25% of shed blood volume of either 0.7% or 8% alginate solution or 5% HES solution. Respective viscosities were 7.6 cP, 10.2 cP and 2.1 cP. All solutions provided resuscitation during the initial 5 min, but only the alginate solutions sustained this for 90 min, while the HES solution returned systemic and microvascular conditions to near shock values. Plasma viscosities for the alginate solutions were 2.1 and 2.6 cP respectively, and 1.1 cP for the HES solution [25].
PEG-Albumin vs. PEG-Alb plus red blood cells
Comparison of viscosity alone vs. the contribution of oxygen carrying capacity provided by RBCs was investigated in hamsters were subjected to 50% hemorrhage resuscitated with 25% of blood volume with solutions containing 6% PEG-albumin only (PEG-BSA 0), and 6% PEG-BSA mixed with autologous RBCs to reach 4 g/dl of hemoglobin (PEG-BSA 4) and 8 g/dl of hemoglobin (PEG-BSA 8). PEG-BSA (6%) had a viscosity of 4.2 cP and a COP of 116 mmHg [26]. Microhemodynamics and tissue pO2 were assessed in the hamster chamber window preparation with intravital microscopy. Arterial base excess tended to be lower than baseline for PEG-BSA 0 and PEG-BSA 4 (ns), whereas base deficit remained significantly decreased for PEG-BSA 8 (p < 0.05 vs. baseline). Oxygen extraction was 91 ± 2 % of the oxygen delivery for PEG-BSA 0 compared to 85 2 % for PEG-BSA 8 (p < 0.05), and FCD was 61%, 47% and 45% for PEG-BSA 0 (p < 0.05 vs. other groups), PEG-BSA 4 and PEG-BSA 8, respectively. The net result was that arterial base excess and oxygen extraction ratio in the tissue was better restored if a higher fraction of PEG-BSA and less RBCs were used. This result suggests that the transfusion trigger in hemorrhagic shock may be shifted towards lower hemoglobin concentrations if highly viscous and oncotic solutions are used.
Effects on tissue oxygen uptake
The hamster window chamber model allows measuring all parameters necessary for determining oxygen tissue uptake. This is accomplished by measuring the local arteriolar blood inflow and venular outflow, blood pO2 and local hematocrit. This data in combination with information on the oxygen dissociation curve for hemoglobin and the plasma oxygen carrying capacity allows determining how a given modification of the composition of blood changes oxygen delivery to the tissue and oxygen uptake (or extraction).
This technique has shown the relative merits of using 4% PEG-Hb (Sangart, San Diego, CA) in resuscitation from a standard shock procedure vs. shed blood and 10% hydroxyethyl starch HES 200 kDa (Pentaspan, B. Braun, Irvine, CA) [27]. In these experiments tissue pO2 was respectively 8 ± 3 mmHg for PEG-Hb, 5 ± 2 mmHg for HES and 19 ± 6 mmHg for shed blood. However oxygen extraction was respectively 4.51, 0.62 and 3.26 ml O2/unit time unit tissue, while total hemoglobin at the time of measurement (60 minutes after resuscitation) was respectively 7.5 ± 0.3, 7.5 ± 1.2 and 10.4 ± 0.5 g/dL. These results sow that improved FCD and capillary perfusion compensates for the decreased oxygen carrying capacity and that even though tissue oxygen is lower relative to using blood the risk for anoxia is similar or may be even lower, considering that more oxygen is delivered per unit time with the low p50 material. Notably even though HES and PEG-Hb had the same total oxygen carrying capacity, the presence of a fraction of hemoglobin with very low p50 significantly improved all oxygen related parameters. This effect is probably due to the oxygen reserve created by low p50 hemoglobin which conserves oxygen and releases this only in localities of very low pO2.
The role of RBC related oxygen carrying capacity in hemorrhage resuscitation
The preceding results converge to the hypothesis that improved resuscitation may be related to the restoration of both blood and plasma viscosity. These two factors are intertwined, however it is possible to increase plasma viscosity at the expense of the increase of blood viscosity, given the strong dependence of blood viscosity on hematocrit. These considerations led to devise an experimental approach by which resuscitation is implemented by the reinfusion of RBCs whose oxygen carrying capacity was annulled by conversion of their hemoglobin to methemoglobin, or saturation with carbon monoxide.
Shock resuscitation with carbon monoxide (CO) saturated blood
The response to the transfusion with CO saturated RBCs on microvascular function and providing in hemorrhagic shock resuscitation was investigated in the conventional of 50% of BV decreases, and restoration one hour after hemorrhage with a single volume infusion of 25% BV with fresh RBCs saturated or unsaturated with CO suspended in HAS [28]. Systemic and microcirculatory restoration were the same for resuscitation with or without carboxyhemoglobin initially (5 – 10 min) up to 90 min after resuscitation. Carboxyhemoglobin concentration decreased over 90 min, increasing oxygen carrying capacity and gradually reoxygenating the tissue.
Shock resuscitation with methemoglbin RBCs (MetRBC)
Some of the beneficial effects noted with CO-hemoglobin RBCs could be attributed to the systemic and microvascular flow improvements noted following top load infusions of CO-saline reported by Hangai-Hoger et al., 2007 [29]. To explore this possibility oxygen carrying capacity was inactivated by converting RBC hemoglobin to methemoglobin by exposure to nitrate. Resuscitation was implemented with fresh RBCs used in the standard shock model as in previous experimental approaches. Results were directly compared to either fresh plasma or blood. Blood viscosities at the end of the 90 min period were 2.4 cP after resuscitation with plasma and 2.9 – 3.0 cP after blood transfusion (baseline: 4.2 cP). Resuscitation with RBCs with or without oxygen carrying capacity had greater mean arterial pressure than the plasma resuscitation group. FCD was substantially higher for RBC transfusions (56 ± 7 % of baseline) vs. plasma (46 ± 7 % of baseline), and the use of MetRBC did not change FCD or microvascular hemodynamics compared to OxyHb. As expected oxygen delivery and extraction were significantly lower for resuscitation with plasma and MetRBCs compared to oxygen carrying RBCs. Systemic and microvascular conditions after volume restitution with plasma were notably worse than either form of RBC related recovery [30].
These studies show that restoration of vascular homeostasis during hemorrhagic shock can be achieved by volume restoration with a fluid with rheological properties similar to those of blood, independently of oxygen carrying capacity. This explains in part why a previous study [31] showed that transfusions of stored RBCs, which do not necessarily raise the effective capacity of blood to transport oxygen upon transfusion, still provided beneficial effects, since RBCs, whether oxygen carrying or not restore perfusion, which is a crucial factor for allowing oxygen delivery by the remaining RBCs and flushing out metabolites produced during shock state. A physiological mechanism behind the restoration of blood viscosity is its direct link to the increase in shears stress in the circulation, and therefore the production of nitric oxide NO by the endothelium. The curtailed recovery of microvascular function appears to be due to the decrease in blood viscosity. Conversely restoration of blood rheological properties improves resuscitation independently of the restitution of oxygen carrying capacity.
Non oxygen carrying red blood cells and high viscosity plasma expanders have similar viscosities and provide similar significant improvements in shock resuscitation. However it cannot be exclude that freshly harvested and transfused blood red blood cells improve resuscitation via the additional mechanism of releasing ATP, since hypoxic red blood cells can release ATP causing vasodilation, a mechanism that could be present even in the presence of methemoglobin in red cells [32].
Hemoglobin vesicles, midway between molecular and cellular hemoglobin
Phospholipid vesicle encapsulating hemoglobin (Hb vesicle, HbV) have been developed to introduce hemoglobin into the circulation in conditions similar to those existing in RBCs, whereby a membrane permeable to oxygen encloses molecular Hb, thus providing oxygen carrying capacity to plasma with hemoglobin concentrations much greater than those that can be practically used when the protein is dissolved in plasma. This material was developed at Waseda University by the group of Prof. Tsuchida, and has the additional feature that the phospholipid membrane is conjugated with PEG, which eliminates the tendency of vesicles to aggregate, thus providing viscogenic properties similar to those of blood, at comparable hemoglobin concentrations. This material was evaluated in our standard shock model in the hamster window chamber preparation [33]. It was delivered as a resuscitation fluid formulated in a 8% human serum albumin (HSA) suspension at Hb concentrations of 3.8 g/dL [HbV(3.8)/HSA] and 7.6 g/dL [HbV(7.6)/HSA]. The viscosity of PEG-Hemoglobin vesicles at 10% concentration by volume in 5 % HAS is 3.7 cP [34]. Hamsters received either HbV(3.8)/HSA or HbV(7.6)/HSA suspensions and restored mean arterial blood pressure to 93 ± 14 and 93 ± 10 mmHg, respectively, similar to those receiving shed blood (98 ± 13 mmHg) and significantly higher than with resuscitation with HSA alone (62 ± 12 mmHg). In general recovery of microvascular blood flow was similar to that obtained by resuscitation with shed blood, notably only for the lower concentration of encapsulated hemoglobin. FCD recovered to approximately 50% of control conditions, while shed blood provided full recovery of this parameter.
These results suggest that while Hb vesicles optimize oxygen delivery capacity, the formulation in a viscogenic medium that allows to simultaneously exploiting the intrinsic high oxygen carrying and delivery capacity and the inherent absence of vasoactivity, may not be fully exploited at this time.
The role of oxygen transport parameters in an effective oxygen carrying blood substitute
The preceding summary of experimental results has the unique feature of comparing several strategies for resuscitation, simultaneously at the systemic and microvascular level, in a model where the tissue is isolated from the environment, and data is obtained without anesthesia.
Analysis at the microvascular level shows that FCD is a critical parameter that portrays how the tissue is supported by the circulation in the resuscitation process that cannot be inferred from systemic evaluation of mean arterial blood pressure. It is important to consider that maintenance of FCD requires maintenance of capillary pressure, since capillaries narrow when this is reduced [6]. Paradoxically adequate capillary pressure is insured by the unrestricted transmission of central pressure to the periphery, a conditions that that implies some level reduction of peripheral vascular resistance or vasodilatation. Clearly in none of the resuscitation procedures studied, even those including blood, was blood viscosity returned to normal values, however it was significantly increased relative to that attained with conventional low viscosity plasma expanders such as dextran 70 kDa and Ringer’s lactate. Hemorrhagic shock reduces heart function due to the limitation of oxygen transport capacity and delivery to the heart muscle due to lowered circulating blood volume and lowered intrinsic blood oxygen carrying capacity. Restoring blood volume is the universal first approach to recovery, however our results show that restoration of oxygen carrying capacity can be postponed if adequate microvascular function is maintained, presumably also in the heart. Whether these effects do take place in the heart is not presently known, however, measurements of cardiac index in extreme hemodilution (anemia) show that the use viscogenic PEG-colloids significantly improves cardiac index by comparison to conventional low viscosity colloids [35].
Conclusions reached using the results in the hamster window chamber model are a simplification of the resuscitation process since there is no direct information of events in the major organs. However in conditions of hemorrhagic shock the circulation tends to centralize, prioritizing redistribution of flow to the major organs at the expense of the periphery. Therefore re-establishment of microvascular function in the tissue of the window chamber (comprising skeletal muscle, adipose and connective tissue) suggests the recovery of central circulatory conditions to the extent the additional requirements of the periphery can be in part or completely satisfied. Support for this contention is provided by comparing the recovery of base excess with the different fluids (Table 1). It should also be noted that the experimental model is designed to analyze hemorrhage and resuscitation conditions for which the different oxygen carrying and non carrying plasma expanders were developed. In this context it is not particularly informative for analyzing conditions of exercise leading to increased oxygen consumption and probably more tightly regulated capillary perfusion.
Table 1.
Microvascular and systemic parameters in resuscitation of startdard hemorrhagic shock 60 minutes after start of resuscitation
| Fluid | 5% HES [27] | 20% HES [23] | Alginate 0.8 [25] | PBH [20] | PEG-Hb [27] | Shed blood [27] |
|---|---|---|---|---|---|---|
| Solution viscosity, cP | 2.0 | 14.4 | 10.2 | 2.0 | 2.4 | 4.5 |
| Plasma viscosity, cP | 0.9 | 0.98 | 2.6 | n.a. | 1.5 | n.a. |
| Mean arterial pressure, mmHg | 57 ± 10 | 95 ± 8 | 98 ± 4 | 101 ± 14 | 90 ± 11 | 79 ± 10 |
| B.E. mmol/L | −0.3 ± 5.7 | 3.2 ± 2.5 | −1.3 ± 2.5 | 1.6 ± 3.4 | 5.4 ± 4.7 | 1.7 ± 3.8 |
| FCD, % | 32 | 52 | 62 | 44 | 66 | 44 |
| Arteriolar O2 delivery relative to control | 0.68 | 1.05 | n.a. | 1.14 | 5.94 | 4.25 |
| O2 extraction/delivery | 0.91 | 0.91 | n.a. | 0.82 | 0.76 | 0.77 |
| Tissue pO2, mmHg | 5 ± 2 | 5 – 6 ± 4 | n.a | 5 ± 2 | 8 ± 3 | 19 ± 6 |
| Total Hb, g/dL | 7.5 ± 1.2 | 6.3 ± 0.4 | 8.3 ± 0.6 | 8.5 ± 0.7 | 7.5 ± 0.3 | 9.3 ± 0.4 |
The shock level attained in this experimental model is admittedly not extreme, since all animals survive, regardless of the procedure used. This is a matter of experimental logistics, since if the shock level is sufficiently severe, resuscitation with the less effective fluids would not lead to survival, and therefore prevent analyzing the functional mechanism at play in the different resuscitation processes. In this context we studied also an extreme shock model, which extended the untreated shock period to 4 hours. This model showed that in hemorrhagic shock survival is determined by maintenance of FCD and independent of tissue oxygenation [8].
Regarding the development of blood substitutes these studies show that either PEG albumin or hemoglobin modifications yield better resuscitation outcomes. Notably this occurs at comparably low plasma concentrations, which in general do not exceed about 1 g/dL. Both the oxygen carrying and non carrying materials have the property of re-establish microvascular flow to near normal levels. The physiological mechanism behind this effect is not yet precisely established, particularly since it is common to both the oxygen carrying and non carrying compounds. However microvascular studies support the concept that it should be a property related to the PEG conjugation with either albumin or hemoglobin. PEG conjugated albumin increases plasma viscosity to some extent, but not as much as alginates, high molecular weight dextrans, or concentrated HES solutions all of which have the same physiological effect of increasing microvascular flow.
It is apparent that in terms of oxygen transport the improvement and maintenance of near normal microvascular blood flow is synergistic with the high affinity of PEG-Hb. There is presently evidence that over oxygenation of the arteriolar wall leads to vasoconstriction [36]. It may also lead to increased oxygen consumption by the arteriolar wall, without a direct benefit to overall tissue oxygenation [37, 38]. Therefore a high affinity oxygen carrier would lower oxygen delivery to oxygenated areas such as arteriolar walls, while reserving delivery to the low tissue pO2 districts, an effect mostly confirmed by the high oxygen delivery found with the comparatively low oxygen carrying capacity increase in the use of PEG-Hb in resuscitation.
Conclusions
Experimentation in a single model using a variety of resuscitation fluids with oxygen carrying and non carrying transport properties shows that there is a significant benefit in the proper management of blood viscosity in resuscitation from hemorrhagic shock. Conventional plasma expanders cause early microvascular impairment because their administration for the purpose of restoring blood volume significantly lowers blood viscosity. Maintenance of blood viscosity postpones the need for correcting the loss of intrinsic oxygen carrying capacity via a either blood or a molecular hemoglobin based blood substitute. This effect is due to blood viscosity increasing peripheral vascular resistance without causing vasoconstriction, resulting in the maintenance of adequate FCD even when systemic blood pressure is not fully re-established. In this context the underlying cause to the decision of transfusing blood upon reaching the “transfusion trigger” appears to be also based on symptoms related to a deficit of uniform tissue perfusion that lead to focal ischemia rather than the lack of oxygen carrying capacity.
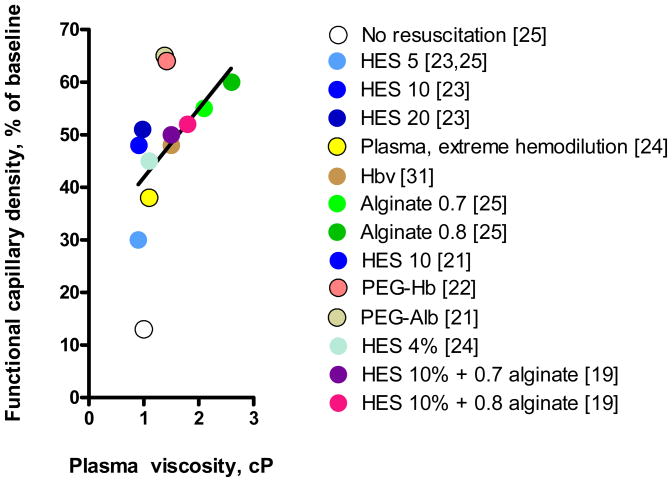
The outcome of managing plasma viscosity in hemorrhagic shock is illustrated in Figure 1 showing the trend emerging for the experimental program centered on microvascular studies reported in this paper. The data shows the results cited in each of the interventions described. Clearly this is only a trend, without statistical significance, however it shows that under the premise that FCD is the critical tissue parameter for resuscitation, its recovery is most likely to occur at high plasma viscosities rather that low ones.
Figure 1.
Functional capillary density attained during the resuscitation from hemorrhagic shock with different oxygen carrying and non carrying colloidal solutions as a function of the plasma viscosity. It is apparent that the higher plasma viscosities uniformly improve microvascular function independently whether the material carries oxygen. The line shown is indicative of the trend of the data. Numbers in brackets indicate the corresponding reference.
An important oxygen transport property of hemoglobin is its oxygen affinity, and current findings suggest that high oxygen affinity provides “targeted oxygen delivery” to the anoxic areas, bypassing the oxygenated tissue. This has the net effect of lowering the need for a full restoration of blood intrinsic oxygen carrying capacity, since the high affinity carrier unloads oxygen preferentially in the tissue fraction at risk of anoxia. Finally, lack of vasoconstriction associated with the use of PEG conjugated proteins and high viscosity materials, may also have a component of vasodilatation related to shears stress the microvascular wall, and the possible management of local nitric oxide production and transport, therefore it is likely that an evolution in the design of blood substitutes will involve the understanding of transport of nitric oxide and other gases beyond oxygen.
Acknowledgments
This work has been supported by grants R01-HL76182 to AGT, R24-64395, R01-62354 and R01-62318 to MI, 1 P01 HL71064 to J.M. Friedman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
disclosure
M. Intaglietta has an economic interest in Sangart Inc.
Bibliography
- 1.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 2.Cokelet GR. The Rheology and Tube Flow of Blood. McGraw-Hill; New York: 1987. [Google Scholar]
- 3.Messmer K. Hemodilution. Surg Clins N Am. 1975;55:659–678. doi: 10.1016/s0039-6109(16)40641-9. [DOI] [PubMed] [Google Scholar]
- 4.Martini J, Carpentier B, Chavez Negrete A, Frangos JA, Intaglietta M. Paradoxical hypotension following increased hematocrit and blood viscosity. Am J Physiol Heart Circ Physiol. 2005;289:H2136–2143. doi: 10.1152/ajpheart.00490.2005. [DOI] [PubMed] [Google Scholar]
- 5.Friesenecker BE, Tsai AG, Martini J, Ulmer H, Wenzel V, Hasibeder WR, Intaglietta M, Dunser MW. Arteriolar vasoconstrictive response: comparing the effects of arginine vasopressin and norepinephrine. Crit Care. 2006;10:R75. doi: 10.1186/cc4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low and high plasma viscosity expanders. Am J Physiol. 2004;287:H363–H373. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 7.Fagrell B. The skin microcirculation and the pathogenesis of ischaemic necrosis and gangrene. Scand J Clin Lab Invest. 1977;37:473–476. doi: 10.3109/00365517709101834. [DOI] [PubMed] [Google Scholar]
- 8.Kerger H, Saltzman DJ, Menger MD, Messmer K, Intaglietta M. Systemic and subcutaneous microvascular pO2 dissociation during 4-h hemorrhagic shock in conscious hamsters. Am J Physiol. 1996;270:H827–H836. doi: 10.1152/ajpheart.1996.270.3.H827. [DOI] [PubMed] [Google Scholar]
- 9.Tsai AG, Friesenecker B, McCarthy M, Sakai H, Intaglietta M. Plasma viscosity regulates capillary perfusion during extreme hemodilution in hamster skin fold model. Am J Physiol. 1998;275:H2170–H2180. doi: 10.1152/ajpheart.1998.275.6.H2170. [DOI] [PubMed] [Google Scholar]
- 10.Cabrales P, Tsai AG, Intaglietta M. Increased tissue pO2 and decreased O2 delivery and consumption after 80% exchange transfusion with polymerized hemoglobin. Am J Physiol. 2004;287:H2825–2833. doi: 10.1152/ajpheart.00654.2004. [DOI] [PubMed] [Google Scholar]
- 11.Tsai AG, Johnson PC, Intaglietta M. Is the distribution of tissue pO2 homogenous? Antioxid Redox Signal. 2007 doi: 10.1089/ars.2007.1633. In press. [DOI] [PubMed] [Google Scholar]
- 12.Kerger H, Groth G, Kalenka A, Vajkoczy P, Tsai AG, Intaglietta M. pO2 measurements by phosphorescence quenching: characteristics and applications of an automated system. Microvasc Res. 2003;65:32–38. doi: 10.1016/s0026-2862(02)00027-4. [DOI] [PubMed] [Google Scholar]
- 13.Meyer JU, Lindbom L, Intaglietta M. Coordinated diameter oscillations at arteriolar bifurcations in skeletal muscle. Am J Physiol. 1987;253:H568–573. doi: 10.1152/ajpheart.1987.253.3.H568. [DOI] [PubMed] [Google Scholar]
- 14.Chance B, Oshino N, Sugano T, Mayevsky A. Basic principles of tissue oxygen determination from mitochondrial signals. Adv Exp Med Biol. 1973;37A:277–292. doi: 10.1007/978-1-4684-3288-6_35. [DOI] [PubMed] [Google Scholar]
- 15.Richmond KN, Shonat RD, Lynch RM, Johnson PC. Critical pO2 of skeletal muscle in vivo. Am J Physiol. 1999;277:H1831–H1840. doi: 10.1152/ajpheart.1999.277.5.H1831. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal M, Martel D, LaManna JC, Jobsis FF. In situ studies of oxidative energy metabolism during transient cortical ischemia in cats. Exp Neurol. 1976;50:477–494. doi: 10.1016/0014-4886(76)90020-0. [DOI] [PubMed] [Google Scholar]
- 17.Nishiki K, Erecinska M, Wilson DF. Effect of Amytal on metabolism of perfused rat heart: relationship between glycolysis and oxidative phosphorylation. Am J Physiol. 1979;237:C221–230. doi: 10.1152/ajpcell.1979.237.5.C221. [DOI] [PubMed] [Google Scholar]
- 18.Kerger H, Tsai AG, Saltzman DJ, Winslow RM, Intaglietta M. Fluid resuscitation with O2 vs. non-O2 carriers after 2 h of hemorrhagic shock in conscious hamsters. Am J Physiol. 1997;272:H525–H537. doi: 10.1152/ajpheart.1997.272.1.H525. [DOI] [PubMed] [Google Scholar]
- 19.Burhop K, Gordon D, Estep T. Review of hemoglobin-induced myocardial lesions. Artif Cells Blood Substit Immobil Biotechnol. 2004;32:353–374. doi: 10.1081/bio-200027429. [DOI] [PubMed] [Google Scholar]
- 20.Wettstein R, Tsai AG, Harder Y, Erni D, Intaglietta M. Early resuscitation with polymerized bovine hemoglobin reverses acidosis, but not peripheral tissue oxygenation, in a severe hamster shock model. Shock. 2006;26:496–503. doi: 10.1097/01.shk.0000228793.87678.55. [DOI] [PubMed] [Google Scholar]
- 21.Cabrales P, Nacharaju P, Manjula BN, Tsai AG, Acharya SA, Intaglietta M. Early difference in tissue pH and microvascular hemodynamics in hemorrhagic shock resuscitation using polyethylene glycol-albumin- and hydroxyethyl starch-based plasma expanders. Shock. 2005;24:66–73. doi: 10.1097/01.shk.0000167111.80753.ef. [DOI] [PubMed] [Google Scholar]
- 22.Wettstein R, Cabrales P, Erni D, Tsai AG, Winslow RM, Intaglietta M. Resuscitation from hemorrhagic shock with MalPEG-albumin: Comparison with MalPEG-hemoglobin. Shock. 2004;22:351–357. doi: 10.1097/01.shk.0000135253.14076.d9. [DOI] [PubMed] [Google Scholar]
- 23.Wettstein R, Erni D, Intaglietta M, Tsai AG. Rapid restoration of microcirculatory blood flow with hyperviscous and hyperoncotic solutions lowers the transfusion trigger in resuscitation from hemorrhagic shock. Shock. 2006;25:641–646. doi: 10.1097/01.shk.0000209532.15317.15. [DOI] [PubMed] [Google Scholar]
- 24.Cabrales P, Intaglietta M, Tsai AG. Increase plasma viscosity sustains microcirculation after resuscitation from hemorrhagic shock and continuous bleeding. Shock. 2005;23:549–555. [PubMed] [Google Scholar]
- 25.Cabrales P, Tsai AG, Intaglietta M. Hyperosmotic-hyperoncotic vs. hyperosmotic-hyperviscous small volume resuscitation in hemorrhagic shock. Shock. 2004;22:431–437. doi: 10.1097/01.shk.0000140662.72907.95. [DOI] [PubMed] [Google Scholar]
- 26.Wettstein R, Tsai AG, Erni D, Lukyanov AN, Torchilin VP, Intaglietta M. Improving microcirculation is more effective than substitution of red blood cells to correct metabolic disorder in experimental hemorrhagic shock. Shock. 2004;21:235–240. doi: 10.1097/01.shk.0000114301.36496.ea. [DOI] [PubMed] [Google Scholar]
- 27.Wettstein R, Tsai AG, Erni D, Winslow RM, Intaglietta M. Resuscitation with MalPEG-Hemoglobin improves microcirculatory blood flow and tissue oxygenation after hemorrhagic shock in awake hamsters. Crit Care Med. 2003;31:1824–1830. doi: 10.1097/01.CCM.0000069340.16319.F2. [DOI] [PubMed] [Google Scholar]
- 28.Cabrales P, Tsai AG, Intaglietta M. Hemorrhagic shock resuscitation with carbon monoxide saturated blood. Resuscitation. 2007;72:306–318. doi: 10.1016/j.resuscitation.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Hangai-Hoger N, Tsai AG, Cabrales P, Suematsu M, Intaglietta M. Microvascular and systemic effects following top load administration of saturated carbon monoxide-saline solution. Crit Care Med. 2007;35:335–237. doi: 10.1097/01.CCM.0000259533.84180.C7. [DOI] [PubMed] [Google Scholar]
- 30.Cabrales P, Tsai AG, Intaglietta M. Is resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity or blood viscosity? Shock. 2007;27:380–389. doi: 10.1097/01.shk.0000239782.71516.ba. [DOI] [PubMed] [Google Scholar]
- 31.Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored RBCs in normovolemic anemic conditions. Transfusion. 2004;44:1626–1634. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 32.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab. 2007;18:350–355. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Sakai H, Takeoka S, Wettstein R, Tsai AG, Intaglietta M, Tsuchida E. Systemic and microvascular responses to hemorrhage shock and resuscitation with Hb vesicles. Am J Physiol Heart Circ Physiol. 2002;283:H1191–H1199. doi: 10.1152/ajpheart.00080.2002. [DOI] [PubMed] [Google Scholar]
- 34.Sakai H, Tsai AG, Kerger H, Park SI, Takeoka S, Nishide H, Tsuchida E, Intaglietta M. Subcutaneous microvascular responses to hemodilution with a red cell substitute consisting of polyethyleneglycol-modified vesicles encapsulating hemoglobin. J Biomed Mater Res. 1998;40:66–78. doi: 10.1002/(sici)1097-4636(199804)40:1<66::aid-jbm8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 35.Cabrales P, Tsai AG, Winslow RM, Intaglietta M. Extreme hemodilution with PEG-hemoglobin vs. PEG-albumin. Am J Physiol. 2005;289:H2392–2400. doi: 10.1152/ajpheart.00225.2005. [DOI] [PubMed] [Google Scholar]
- 36.Cabrales P, Tsai AG, Intaglietta M. Nitric oxide regulation of microvascular oxygen exchange during hypoxia and hyperoxia. J Appl Physiol. 2006;100:1181–1187. doi: 10.1152/japplphysiol.01105.2005. [DOI] [PubMed] [Google Scholar]
- 37.Tsai AG, Cabrales P, Winslow RM, Intaglietta M. Microvascular oxygen distribution in awake hamster window chamber model during hyperoxia. Am J Physiol Heart Circ Physiol. 2003;285:H1537–H1545. doi: 10.1152/ajpheart.00176.2003. [DOI] [PubMed] [Google Scholar]
- 38.Tsai AG, Cabrales P, Hangai-Hoger N, Intaglietta M. Oxygen distribution and respiration by the microcirculation. Antioxidants & Redox Signaling. 2004;6:1011–1018. doi: 10.1089/ars.2004.6.1011. [DOI] [PubMed] [Google Scholar]