Table 1.
Borostannylation of Alkynes Using 1 Followed by Dioxaborolidene Formationa
| entry | alkyne | adduct (%yield)b |
|---|---|---|
| 1. |
2a |
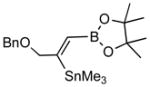 4a (83) |
| 2. |
2b |
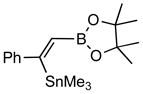 4b (82) |
| 3. |
2c |
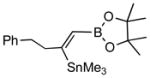 4c (81) |
| 4. |
2d |
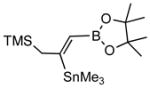 4d (80) |
| 5. |
2e |
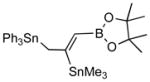 4e (80) |
| 6. |
2f |
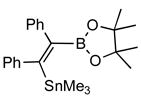 4f (84) |
| 7. |
2g |
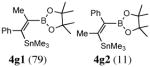 |
| 8. |
2hc |
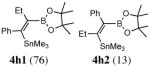 |
see Scheme 1 for procedure.
isolated by column chromatography.
use of PdCl2(CH3CN)2/P(But)3 (60 °C, 12 h) gives a ratio of 4h1: 4h2 = 94:6.
