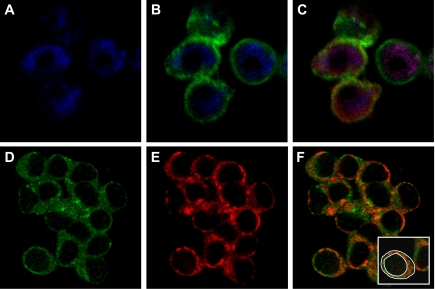
Fig. 3.
Confirmation of endothelial cell isolation from porcine coronary arteries and localization of endothelial nitric oxide synthase (eNOS) and caveolin-1. Endothelial cells were isolated from control arteries of sedentary pigs. Cells were fixed and triple immunolabeled with anti-CD146, anti-eNOS, and anti-caveolin-1, followed by fluorescent-labeled secondary antisera: anti-mouse-IgG-Cy5, anti-rabbit-IgG-FITC, and anti-mouse-IgM-Rhodamine Red X for detection of anti-CD146, -eNOS, and -caveolin-1, respectively. FITC, Rhodamine Red X, or Cy5 was excited with a 15-mW Kr-Ar laser using the 488-, 568-, and 647-nm bands, respectively. FITC, Rhodamine Red X, or Cy5 was detected through a DF522/35 filter, HQ598/40 band-pass filter, or DF522/35 filter, respectively. A: cells stained for CD146 (blue pixels), a marker specific for endothelial cells. B: superimposition of simultaneously acquired signals from anti-CD146 (blue pixels) and anti-eNOS (green pixels) further confirm the isolation of endothelial cells. C: superposition of simultaneously acquired signals from anti-CD146 (blue pixels), anti-eNOS (green pixels), and anti-caveolin-1 (red pixels). D: cellular images indicate that eNOS (green pixels) is primarily localized to the plasma membrane with some cytosolic distribution in freshly isolated endothelial cells. E: similarly, caveolin-1 (red pixels) was primarily distributed at the plasma membrane with some cytosolic distribution. F: superimposition of simultaneously acquired images of eNOS and caveolin-1 indicate prominent colocalization of these proteins at the plasma membrane. As illustrated (inset), the intensity of total (outer white circle) and plasma membrane-associated (inner white circle) green or red pixels for each cell was separately measured as described in materials and methods.