Abstract
Classical numerical models have attributed the regulation of normal cardiac automaticity in sinoatrial node cells (SANCs) largely to G protein-coupled receptor (GPCR) modulation of sarcolemmal ion currents. More recent experimental evidence, however, has indicated that GPCR modulation of SANCs automaticity involves spontaneous, rhythmic, local Ca2+ releases (LCRs) from the sarcoplasmic reticulum (SR). We explored the GPCR rate modulation of SANCs using a unique and novel numerical model of SANCs in which Ca2+-release characteristics are graded by variations in the SR Ca2+ pumping capability, mimicking the modulation by phospholamban regulated by cAMP-mediated, PKA-activated signaling. The model faithfully predicted the entire range of physiological chronotropic modulation of SANCs by the activation of β-adrenergic receptors or cholinergic receptors only when experimentally documented changes of sarcolemmal ion channels are combined with a simultaneous increase/decrease in SR Ca2+ pumping capability. The novel numerical mechanism of GPCR rate modulation is based on numerous complex synergistic interactions between sarcolemmal and intracellular processes via membrane voltage and Ca2+. Major interactions include changes of diastolic Na+/Ca2+ exchanger current that couple earlier/later diastolic Ca2+ releases (predicting the experimentally defined LCR period shift) of increased/decreased amplitude (predicting changes in LCR signal mass, i.e., the product of LCR spatial size, amplitude, and number per cycle) to the diastolic depolarization and ultimately to the spontaneous action potential firing rate. Concomitantly, larger/smaller and more/less frequent activation of L-type Ca2+ current shifts the cellular Ca2+ balance to support the respective Ca2+ cycling changes. In conclusion, our model simulations corroborate recent experimental results in rabbit SANCs pointing to a new paradigm for GPCR heart rate modulation by a complex system of dynamically coupled sarcolemmal and intracellular proteins.
Keywords: calcium, sarcoplasmic reticulum, ion channels, numerical modeling, sinoatrial node cell
theoretical modeling is an approach to unravel the complexity of cardiac pacemaker function. As early as 1960, a quantitative theory of membrane excitation by Hodgkin and Huxley (H-H) was modified to simulate spontaneous action potentials (APs) of cardiac pacemaker cells (39). Formulation of the 1960 model was a case of “theory leading experiment” (40). Numerous models of the primary cardiac pacemaker cell, the sinoatrial node cell (SANC), have been developed [12 models since 1980 (54)] based on the H-H formalism and extensive studies of sarcolemmal ion currents under voltage clamp. Accordingly, the regulation of the spontaneous SANC rate has been described so far based largely on modulatory changes of ion channels, such as “funny” current (If), L-type Ca2+ current (ICaL), steady current (Ist), and acetylcholine-activated K+ current (IKACh) (6, 12, 18, 58). Although the relative contributions of each channel type have been always debated (26, 40, 54), until very recently the dominant point of view was that If, sometimes called “the pacemaker current,” provides the major mechanism for heart rate regulation via its voltage activation shifts modulated by cAMP (9, 10). The most recent studies in genetically manipulated mice demonstrated, however, that the β-adrenergic receptor (β-AR) stimulation effect is preserved in the absence of If (16) or in the absence of cAMP sensitivity of If (1).
Thus, the SANC pacemaker system is likely more complex than just a H-H-type membrane voltage oscillator (i.e., a membrane clock or M clock) and includes additional critical contributions that have not been currently described by the existing numerical models of autonomic modulation of the SANC spontaneous rate (6, 12, 18, 58). Indeed, experimental data of the last two decades have demonstrated that the SANC pacemaker system has a substantial contribution of Ca2+ cycling by the sarcoplasmic reticulum (SR) (for reviews, see Refs. 27 and 34). Specifically, confocal imaging detects not only a global intracellular Ca2+ transient triggered by the AP but also spontaneous local Ca2+ releases (LCRs) from the SR. LCRs emerge beneath the cell membrane as multiple, relatively synchronous, locally propagating Ca2+ wavelets later in the cycle, i.e., during the late part of diastolic depolarization (DD) (3, 21). Importantly, the SR behaves as a true intracellular Ca2+ oscillator (recently dubbed a “Ca2+ clock”), generating rhythmic LCRs observed in voltage-clamped and chemically skinned SANCs (53). Variable periods of the SR Ca2+ oscillator are achieved via gradations in basal cAMP-dependent PKA signaling (50) that regulate the SR Ca2+ pump (via phospholamban phosphorylation) and likely Ca2+-release channels [ryanodine receptors (RyRs)]. LCRs activate the electrogenic operation of the sarcolemmal Na+/Ca2+ exchanger (NCX), generating an inward current (INCX) that imparts a steep, exponentially rising phase to DD (2, 3, 21). Furthermore, robust experimental evidence has indicated that PKA-dependent modulation of LCR characteristics is a major mechanism contributing to the pacemaker rate modulation via β-ARs (rate increase) (2, 49, 50) and cholinergic receptors (ChRs; rate decrease) (32). Specifically, β-ARs or ChRs shorten or prolong the LCR period (time from the peak AP-induced Ca2+ transient to the LCR peak), i.e., LCRs occur earlier or later during DD, respectively. Also, the integrated LCR signal or signal mass (the product of LCR size, amplitude, and number per cycle) concomitantly increases or decreases, respectively.
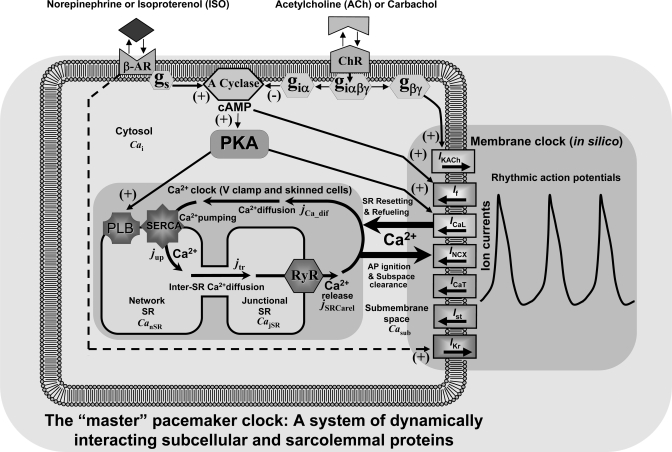
While these results can be interpreted to indicate that G protein-coupled receptor (GPCR) signaling modulates automaticity via the intracellular Ca2+ cycling that entrains rhythmic excitations of cell surface membrane (35, 51), the true mechanisms of GPCR modulation are, in fact, likely complex, and exploration of these complex interactions requires numerical modeling. Indeed, surface membrane proteins not only effect changes in membrane potential but also critically regulate intracellular Ca2+ cycling [e.g., via effecting the global Ca2+ transient, via balancing Ca2+ influx and efflux, via AP shape influencing NCX function, etc. (for a review, see Ref. 27)]. In other words, while either clock, by definition, exists without the other only under special experimental conditions (the Ca2+ clock under voltage clamp or in skinned cells) or in silico (36, 54), in nature, the clocks' mutual entrainment substantially alters the properties of each clock. Therefore, when operating as a system, the membrane clock and Ca2+ clock cease to exist as separate entities: there is only one “master” pacemaker clock (Fig. 1), i.e., a complex oscillatory system of dynamically interacting sarcolemmal and intracellular molecules (27, 36).
Fig. 1.
Schematic illustration of our model of autonomic modulation of a cardiac pacemaker cell system of dynamically interacting subcellular and sarcolemmal proteins. Rate regulation mechanisms were numerically explored for the stimulation of β-adrenergic receptors (β-ARs) or cholinergic receptors (ChRs), to increase or slow rate, respectively. Norepinephrine [or isoproterenol (ISO)] and ACh (or carbachol) bind to their receptors and regulate adenylyl cyclase (A Cyclase) and cAMP levels up and down via their G proteins, Gs and Giα, respectively. Varying cAMP levels, in turn, via cAMP-dependent PKA phosphorylation of phospholamban (PLB) and L-type Ca2+ channels [generating L-type Ca2+ current (ICaL)], couples the function of Ca2+ and membrane (M) clocks, resulting in orchestrated and balanced shifts in their function and interactions to robustly control the pacemaker rate. cAMP-dependent “funny” current (If) provides a safety mechanism at low rates and rate transitions. In addition, ACh slows the system also via classical ACh-activated K+ current (IKACh) mechanism mediated by Gβγ, an effect that becomes dominant at higher levels (supraphysiological) of [ACh]. INCX, Na+/Ca2+ exchanger current; ICaT, T-type Ca2+ current; Ist, steady current; IKr, rapid inward rectifying K+ current; SR, sarcoplasmic reticulum; Cai, cystolic Ca2+; V clamp, voltage clamp; jCa_diff, the Ca2+ diffusion flux from submembrane space to cytosol; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase; jup, rate of SR Ca2+ uptake; jtr, the Ca2+ diffusion flux from the network SR to junctional SR; RyR, ryanodine receptor; jSRCare1, diastolic Ca2+ release; CanSR, CajSR, and Casub, Ca2+ in the network SR, junctional SR, and submembrane space, respectively.
We (36) have recently developed a novel unique numerical model of the SANC that features spontaneous diastolic Ca2+ releases. Both amplitude (mimicking the LCR signal mass) and phase (mimicking the average LCR period) of Ca2+ releases can be graded in the model by gradations in the SR Ca2+ pumping capability, mimicking experimentally defined changes in phospholamban function resulting from gradations in phospholamban phosphorylation by the cAMP-mediated activation of PKA. The present study uses this model to explore a novel quantitative explanation for the autonomic modulation of the spontaneous AP rate of SANCs via β-AR and ChR stimulation.
METHODS
Numerical SANC Model
The present study is based on our recently published “basal state firing model” of rabbit SANCs (36). This numerical model can be downloaded and run in CellML format (http://models.cellml.org/workspace/maltsev_2009). The formulations of the original model (36) were upgraded (Fig. 1) to simulate the effects of stimulation of β-ARs and ChRs as described below. All model details, including mathematical formulations and specific parameter values, are provided in the Supplemental Material.1 All 30 model variables and their initial values are listed in Supplemental Table S1.
Simulation of ICaL in the Basal State
ICaL is a major player in our model: it 1) generates the AP upstroke; 2) contributes to late DD; 3) “resets” Ca2+ cycling by triggering global Ca2+-induced Ca2+ release, resulting in strong SR Ca2+ depletion (36); and 4) “refuels” the SR by supplying (each cycle) SANCs with Ca2+ to be pumped into the SR (36). We used standard formulations for ICaL described by activation and inactivation gating (variables dL and fL) and Ca2+-dependent inactivation (fCa) as previously described (24). A critical parameter determining the ICaL contribution to DD is its activation threshold, and, therefore, this parameter was carefully considered in our model. The activation threshold has been measured in several patch-clamp studies of isolated rabbit SANCs and was found to be “positive to −45 to −50 mV” (8), “around −60 mV” (48), “between −50 and −40 mV” (38), or “approximately −40 mV” (52). A relatively low activation threshold of ICaL in SANCs might be explained, at least in part, by two factors: 1) L-type Ca2+ channels could be phosphorylated by PKA, which is highly active in the basal state (43, 50); and 2) ICaL in SANCs might be a combination of Cav1.2 and Cav1.3, as shown in mouse SANCs (37). Accordingly, we assigned the midpoint of the ICaL steady-state activation curve (36) to −13.5 mV, to be in line with the aforementioned experimental data on the ICaL activation threshold in rabbit SANCs (see Supplemental Table S2 for a comparison with different SANC models). With our settings, the magnitude of the simulated ICaL peak induced by a voltage step to −60 mV (from −80 mV) is negligible (<1 pA), but the actual ICaL activation begins at about −50 mV (3.17 pA or 0.1 pA/pF) and increases to 13.7 pA (0.43 pA/pF) at −40 mV (see the full current-voltage curve in Supplemental Fig. S1). During simulated AP firing, the ICaL magnitude increases from 2.24 pA (0.07 pA/pF) to 8.87 pA (0.277 pA/pF) as DD progresses from −50 to −40 mV (Supplemental Fig. S2).
Specific Formulations for the Modulation of Ca2+ Cycling and Ion Channels by ACh
We included formulations for IKACh that were previously suggested by Demir et al. (6):
| (1) |
where EK is the K+ equilibrium potential, gKACh,max is the maximal conductance of IKACh, and Vm is membrane voltage. The gating variable a(Vm, [ACh], t; where t is time) in this model is governed by an opening rate constant (β) that is [ACh] dependent and a closing rate constant (α) that is voltage dependent:
where β and α are given in 1/ms, [ACh] in mM and Vm in mV. Modulation of If and ICaL by ACh is formulated in the present study as suggested in the 2002 Zhang et al. model (58). In short, the shift (s; in mV) of the If activation curve by ChR stimulation is described by the following Michaelis-Menton-type equation:
| (2) |
where smax = −7.2 mV is the maximum shift of the If activation curve, nf = 0.69 is the Hill coefficient, and K0.5,f = 12.6 nM is the ACh concentration that produces half-maximal shift of the If activation curve. The fractional block (bCaL) of ICaL conductance (gCaL) by ChR stimulation is also calculated using the following Michaelis-Menton-type equation:
| (3) |
where K0.5,CaL = 90 nM. As to the range of ICaL modulation, the experimental data greatly vary: from very strong inhibition by 56%, as reported by Petit-Jacques et al. (43), to no effect, as reported at least in two studies (20, 42). Avoiding the extremes, we assigned a moderate maximum inhibition of ICaL to 31% (bCaL,max = 0.31) based on an experimental report by Zaza et al. (56).
Our model adopts formulations of Luo and Rudy (30) for the rate of SR Ca2+ uptake (i.e., pumping), jup, in the form of the following simple Michaelis-Menton-type function of cytosolic [Ca] (Cai):
| (4) |
where Pup is a maximum uptake rate, i.e., the capability of the SR to pump Ca2+ (at a given state of phospholamban phosphorylation), and Kup is the Cai sensitivity of the SR Ca2+ pump. A major new feature of the present model is that we introduced a dependence of Pup on ChR stimulation, mimicking the effect phospholamban dephosphorylation by ChR activation in rabbit SANCs (32):
| (5) |
where Pup,basal is Pup in the basal state [Pup,basal = 12 mM/s, as in our original “basal state” model (36)] and bup is the fractional block of SR Ca2+ pumping due to phospholamban dephosphorylation. Since PKA simultaneously regulates both phospholamban and L-type Ca2+ channels, we assumed that the inhibition of SR Ca2+ pumping capability by ChR stimulation closely follows the established relationship of the inhibition of ICaL (Eq. 3) (58):
| (6) |
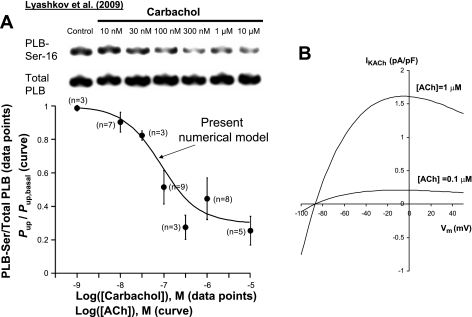
where K0.5,up = K0.5,CaL = 90 nM is the [ACh] for half-maximal inhibition and bup,max = 0.7. The resultant relative decrease of Pup [i.e., Pup ([ACh])/Pup,basal] shown by the solid curve in Fig. 4A (see results for details) closely fits the dose-response relationship of the dephosphorylation of phospholamban at Ser16 previously experimentally measured in rabbit SANCs at graded levels of ChR stimulation (32).
Fig. 4.
A: the modeled relative decrease of the capability of SR Ca2+ pumping, Pup (“Present numerical model” curve) closely fits the relation of PLB dephosphorylation at Ser16 previously experimentally measured in rabbit SANCs at graded levels of ChR stimulation. The curve was calculated as follows: Pup/Pup,basal = 1 − bup([ACh]), where bup is defined according Eq. 6. B: model characterization of IKACh at two ACh concentrations, using the classical IKACh formulation by Demir et al. 1999 (6). IKACh exhibits only a relatively small activation at 100 nM ACh. [All experimental data in A are reproduced from Ref. 32.]
Model Parameters in Simulations of the Effect of β-AR Stimulation
The key membrane parameters were changed in accordance with experimentally documented changes induced by β-AR stimulation by isoproterenol (see details in Supplemental Table 3). Specifically, gCaL was increased by 75% (49). We did not shift the midpoint of the ICaL steady-state activation curve in our simulations of β-AR stimulation because the threshold of ICaL activation remained unchanged in rabbit SANCs after the application of isoproterenol in a thorough experimental study by Zaza et al. (56). The activation curve for If was shifted by 7.8 mV toward positive potentials (10). We increased the maximum conductance (gKr) for the rapid delayed rectifying K+ current (IKr) by 50% because isoproterenol reportedly increases K+ current in rabbit SANCs (29) [although likely via a PKC-dependent mechanism (15)]. In addition to changes of membrane conductances, we also changed the capability of SR Ca2+ pumping, Pup [mimicking the effect of the increased phospholamban phosphorylation (50)]. Since isoproterenol-induced changes of Pup are unknown, we performed a parametric sensitivity analysis for this parameter.
Numerical Integration
Source code for the model was developed using Borland Delphi-7 software. Model integration was performed on a Pentium 4 (2.8 GHz)-based PC with a fixed time step of 0.01 ms.
Steady-State Solutions
We followed the algorithm previously suggested by Kurata et al. (25): numerical integration was continued until the relative differences in both amplitude and period between the newly calculated cycle and the preceding cycle became <0.001.
RESULTS
Prediction of β-AR Stimulation
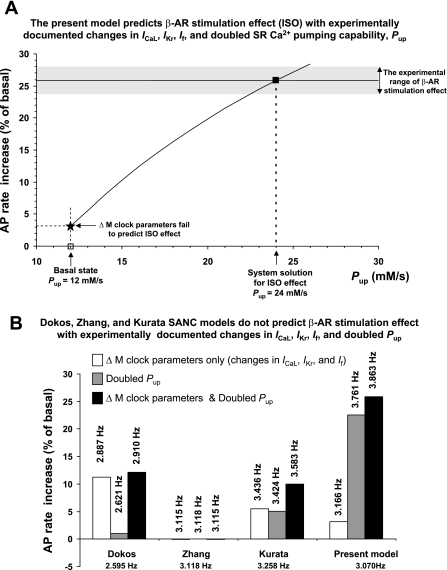
Supplemental Table S3 (“Primary model set”) shows specific parameter changes and numerical results (absolute and relative rate changes) of our simulations of β-AR stimulation. Surprisingly, substantial simultaneous changes of all three major membrane clock components (ICaL, If, and IKr) produced only a moderate 3.15% increase of the AP firing rate (solid star in Fig. 2A) in our modelled SANC system when the capability of SR Ca2+ pumping remained at its basal state of 12 mM/s (left vertical arrow in Fig. 2A). This was far below the β-AR stimulation-induced effect that has been experimentally documented in rabbit SANCs, within 23.7% (49) to ∼28% (14), as marked by the shaded band in Fig. 2A.
Fig. 2.
Present numerical model solution (■ in A) for the SR Ca2+ pumping capability (Pup) to experimentally predict the documented range of β-AR stimulation on the action potential (AP) firing rate (14, 49) (shown by the shaded band). In contrast, previous SANC models, lacking diastolic Ca2+ release, do not predict experimental results (B). Models are labeled as follows: “Dokos”(11), “Zhang” (57), and “Kurata”(24). Respective bars show the effects of changes of M clock parameters, doubling Pup, or both changes combined. M clock changes included a 75% increase of ICaL conductance (gCaL) (49), a 7.8-mV shift of the activation curve for If (10), and a 50% increase of IKr conductance (gKr) (29). See Supplemental Table S3 for more details. The spontaneous rate change was negligible (within 0.1%) in the Zhang et al. model (note that this model has no Ca2+ dynamics, i.e., [Ca2+] = constant). Absolute AP firing rates predicted by the models are shown by the numbers above the respective bars.
Our previous experimental study (50) showed that β-AR stimulation greatly (by a factor of 2.4) increases phospholamban phosphorylation, resulting in an expected increase in Pup. However, the Pup value cannot be directly measured in SANCs and remains unknown. Since previous studies have also shown that Ca2+ releases from the SR critically contribute to β-AR stimulation (49, 50) and that these releases are controlled by SR Ca2+ pumping (50), we tested the hypothesis that graded increases in Pup (solid curve in Fig. 2A), in addition to the aforementioned major ion current changes, would provide a reasonable numerical solution for experimental results with β-AR stimulation. Indeed, a 25.8% rate increase (marked by the solid square in Fig. 2A, i.e., close to the middle of the experimental range) was achieved in the model, with Pup doubling from 12 to 24 mM/s.
We compared our model predictions for β-AR stimulation with those of some previous rabbit SANC models in response to similar changes of their parameters (Fig. 2B). The combined effect of the same relative changes in conductances for ICaL and IKr and the same shift of If activation resulted in a moderate rate change in both the Dokos et al. model [Dokos model (11)] and Kurata et al. model [Kurata model (24)], a result that was similar to our model. Curiously, simulations of 2000 Zhang et al. model of central SANCs [Zhang model (57)] was not sensitive to the combined changes (note that in this particular model intracellular [Ca2+] was originally set to a constant).
In contrast to previous models, our model is highly sensitive to the SR Ca2+ pumping rate (shaded bars in Fig. 2B); doubling Pup, per se, produced a 22.5% rate increase, providing the major contribution to the β-AR stimulation effect. While the Dokos and Kurata models include formulations for Ca2+ dynamics, they do not have terms for diastolic Ca2+ release, and doubling Pup produces a mere 1% and 5% rate increase, respectively (shaded bars in Fig. 2B). Therefore, none of the tested classical models provides a solution for the effect of β-AR stimulation, either with experimentally documented changes in major sarcolemmal ion channels (open bars in Fig. 2B) or with these changes combined with a doubled capability of SR Ca2+ pumping (solid bars in Fig. 2B).
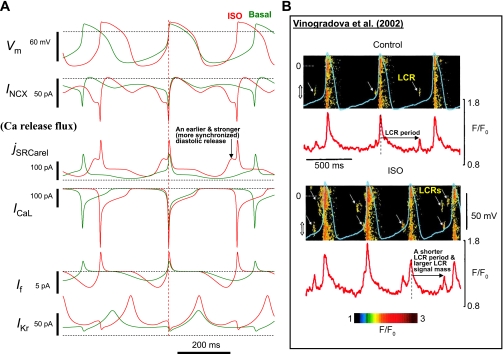
Our numerical mechanism of β-AR stimulation is demonstrated in Fig. 3A, which shows simulated dynamics of major components of the system. In addition to the well-known and expected increases in the amplitudes of ion channel currents (ICaL, If, and IKr), diastolic INCX activation occured earlier in the cycle and doubled in amplitude, due to the earlier occurrence and concomitant strong increase of diastolic Ca2+ release (jSRCarel), respectively. The earlier occurrence of the diastolic Ca2+ release in our model (jSRCarel panel in Fig. 3A) reproduced the documented isoproterenol-induced shortening of the LCR period (50) (horizontal arrows in Fig. 3B). In turn, the stronger diastolic Ca2+ release reproduced the documented isoproterenol-induced increase in LCR signal mass, i.e., the product of LCR spatial size, amplitude, and number per cycle (49) (more frequent and larger-sized LCRs; Fig. 3B).
Fig. 3.
A: simulations showing the novel mechanism of β-AR stimulation to integrate the function of system components. While the major immediate mechanism of cycle length reduction is an earlier and stronger INCX, the stronger and more frequent ICaL critically supports this INCX function by supplying more Ca2+ to the cell and thereby enhancing SR function. Simulated traces are synchronized at the phase of membrane potential (Vm) = 0 (vertical dashed line) to show the earlier and stronger activation of diastolic Ca2+ release (jSRCarel) and INCX, resulting in the earlier and stronger diastolic depolarization(DD) acceleration. B: an example experiment, in which submembrane Ca2+ signals by confocal microscopy and Vm by perforated patch clamp were recorded in rabbit sinoatrial node cells (SANCs) in control and after stimulation of β-AR with ISO. Diastolic local Ca2+ releases (LCRs) are shown by yellow arrows. ISO increases the LCR signal mass and shifts the LCR occurrence to earlier times within the spontaneous cycle. Red curves (below the confocal images) show the time course of spatial averages calculated within the location marked by double-headed arrows along the scanning lines. This important effect is predicted by our model (see the Ca2+ release flux in A, solid vertical arrow). [Images and time series in B were reproduced for illustration from Vinogradova et al. (49) and modified by adding LCR labels and the arrows showing the LCR periods, respectively.]
We also simulated changes in the current-voltage curve for peak ICaL and in subthreshold ICaL dynamics during mid-DD (Supplemental Figs. S1 and S2) and found that ICaL becomes activated earlier and to a greater extent during β-AR stimulation. Interestingly, this apparent ICaL activation shift was linked to a larger gCaL but not to intrinsic activation gating (because the midpoint of the ICaL steady-state activation curve remained unchanged in our simulations; see methods). While the subthreshold ICaL increased during β-AR stimulation, it was still much smaller than INCX during the entire DD, e.g., 3.1 vs. 39 pA at −50 mV and 11.8 vs. 40.9 pA at −40 mV; ICaL and INCX became equal (−44.4 pA) only at −29 mV, i.e., above the ICaL threshold. It is also important to note that Ca2+- and voltage-dependent responses to β-AR stimulation were well balanced within the system: increased Ca2+ influx via increased and more frequent ICaL supported larger SR Ca2+ releases and greater Ca2+ efflux via the doubled INCX.
Prediction of ChR Stimulation
Previous experimental results (32, 47) have shown that the mechanism of SANC rate slowing via ChR activation is complex and includes changes in both sarcolemmal ion currents and diastolic Ca2+ release. Electrophysiological changes include PKA-dependent ICaL reduction (43, 56), a shift of cAMP-dependent If activation toward negative potentials (9, 10), and Gi-dependent activation of IKACh (6, 12) (Fig. 1). The change in diastolic Ca2+ release is mediated, at least in part, by a reduction in cAMP-dependent PKA signaling, resulting in phospholamban dephosphorylation (32), which is expected to decrease the capability of SR Ca2+ pumping (Eqs. 4 –6).
In the rabbit sinoatrial node, ACh application of up to 100 nM decreases the AP rate by up to ∼50%, an effect generally considered as physiological rate regulation (58). Since carbachol, a ChR agonist, decreases the AP rate by ∼50% in rabbit SANCs also at 100 nM (32), its efficacy to stimulate ChRs must be similar to that of ACh, and, therefore, (Eqs. 5 and (6 reasonably describe Pup inhibition at various [ACh] (solid curve in Fig. 4A) based on experimental data of phospholamban dephosphorylation at various carbachol concentrations (data points in Fig. 4A). In line with these experimental results, our model predicted an approximate half-maximal effect (52.6%) of SR Ca2+ pump inhibition at 100 nM [ACh], i.e., a 36.8% in Pup, which is a decrease from its basal state value of 12 to 7.58 mM/s.
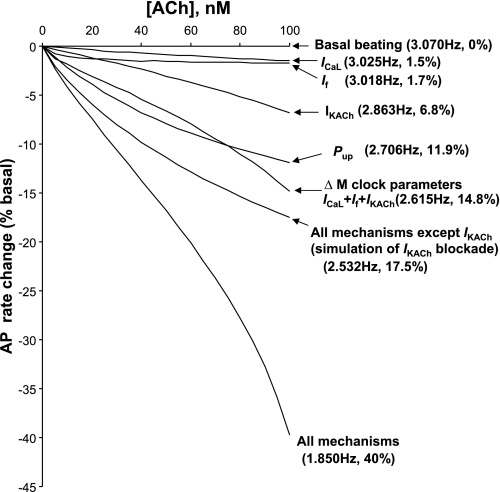
Next, we tested the effects and interactions of all four aforementioned ACh-dependent mechanisms (Pup, ICaL, If, and IKACh) to reduce the AP firing rate in the model in response to ACh within this moderate concentration range (up to 100 nM; Fig. 5; see Supplemental Table 4 for specific model parameters to simulate the effect of ChR stimulation). The model predicted that the basal beating rate substantially reduced by 40% from its basal value (“All mechanisms” curve in Fig. 5), in line with experimental observations of a 50% rate reduction in rabbit SANCs (32). Our simulations showed that the beating rate reduction includes two major mechanisms that act on membrane potential: 1) changes in INCX due to changes in SR Ca2+ cycling and 2) activation of IKACh due to Gi signaling (Figs. 1 and 6A).
Fig. 5.
Synergism of different component mechanisms in moderate SANC rate slowing by [ACh]. Results of model predictions for spontaneous AP rate reduction at various moderate ACh concentrations (up to 100 nM) for different mechanisms (as shown). Modulation (inhibition) of the capability of SR Ca2+ pumping by ACh was described by Eqs. 5 and 6.
Fig. 6.
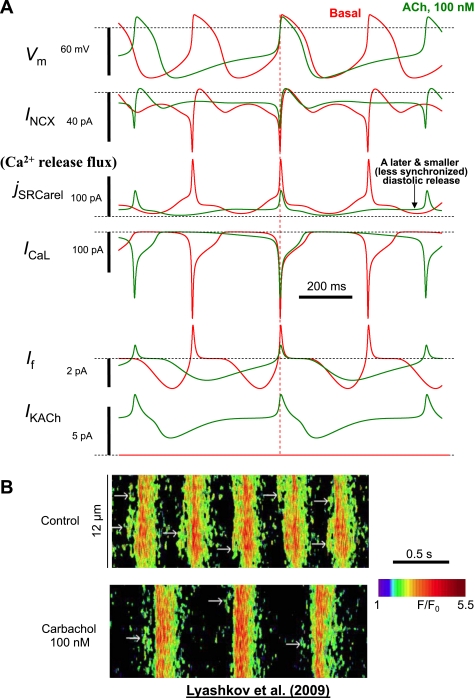
A: simulations of the present model showing the new mechanism of the ChR stimulation effect that integrates function of components of the Ca2+ clock and M clock. While the major immediate mechanism of the cycle length increase is a later and smaller INCX, the smaller and less frequent ICaL critically supports this INCX function by supplying less Ca2+ to the cell and thereby decelerating SR function. Simulated traces are synchronized at the phase of Vm = 0 (vertical dashed line) to show the later and smaller activation of jSRCarel and INCX. B: an example of recording of Casub signals by confocal microscopy in rabbit SANCs in control and after stimulation of ChR with carbachol (100 nM). Carbachol decreased the LCR signal mass and shifted the LCR occurrence to later times within the spontaneous cycle. This important effect is predicted by our model (see Ca2+ release flux in A). [Images in B were reproduced for illustration from our recent study (32).]
The later occurrence of diastolic Ca2+ release in the model simulations reproduced the experimentally documented increase of the LCR period affected by ChR stimulation (Fig. 6B) (32). In turn, the smaller diastolic Ca2+ release reproduced the experimentally documented decrease in LCR signal mass (the product of LCR spatial size, amplitude, and number per cycle; Fig. 6B) (32). In turn, a reduction of Ca2+ influx in the model via smaller and less frequent ICaL came into balance with the smaller Ca2+ releases and reduced Ca2+ efflux via the substantially decreased INCX.
A major contribution of IKACh in the physiological chronotropic effect of ACh at 100 nM (Figs. 5 and 6A) is not surprising. The classical ACh-activated mechanism has been previously well characterized, both experimentally and theoretically (see, e.g., Refs. 6, 32, and 58), and, therefore, it was not explored here in detail. Rather, we simulated the effect of ACh in the presence of IKACh blockade (“All mechanisms except IKACh” curve in Fig. 5). At 100 nM ACh, the effect reached about one-half of the total effect, 17.5% vs. 40%, i.e., consistent with experimental data (32) (see more in the discussion).
This moderate ACh concentration of 100 nM 1) activated only ∼13% of IKACh observed at 1 μM ACh (Fig. 4B, according to Eq. 1); 2) reduced ICaL by 16.3% (Supplemental Fig. S1, about one-half of its maximum modulation range of 31%, see Eq. 3); 3) shifted If activation nearly maximally (activation was shifted by −5.8 mV of the maximum shift of −7.2 mV, see Eq. 2); and 4) reduced Pup from 12 to 7.58 mM/s (Eqs. 5 and 6).
Importantly, the model predicted a relatively small (compared with 40%) reduction of the beating rate produced by each separate mechanism alone at 100 nM ACh (shown by arrows in Fig. 5): ICaL, 1.5%; If, 1.7%, IKACh, 6.8%, and Pup, 11.9%, reflecting a synergism in their combined effects (40%) to slow the rate of the SANC system. Furthermore, changes in membrane ion channels alone (ICaL + If + IKACh) slowed the rate by 14.8%, reflecting a synergism of membrane mechanisms (1.5 + 1.7 + 6.8 = 10 << 14.8) as well as a synergism between the membrane mechanisms and SR Ca2+ cycling (14.8 + 11.9 = 26.7 << 40). Thus, effective (physiological) rate reduction by 40% was achieved in the model via relatively small but synergistic changes of all ACh-dependent system components.
Detailed Numerical Analysis of How β-AR and ChR Stimulation Modulate Spontaneous SR Ca2+ Release in the Submembrane Compartment
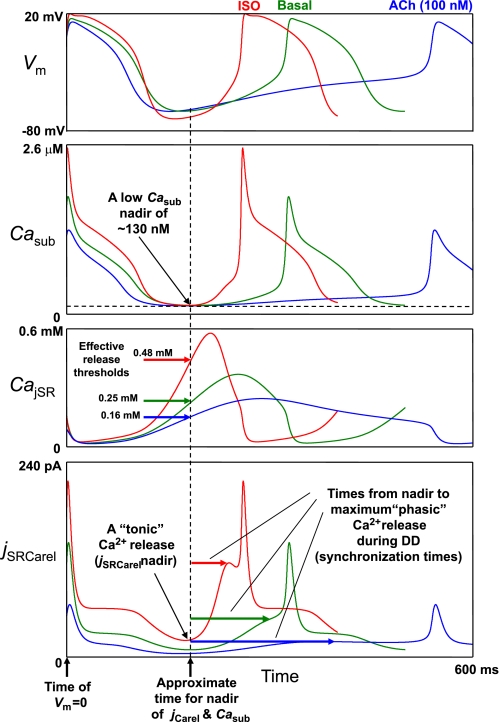
Our mathematical formulation of SR Ca2+ release (jSRCarel) by RyRs in rabbit cardiac cells was adopted from Stern et al. (46) and Shannon et al. (45) (see the Supplemental Material for details). jSRCarel dynamics are governed by the difference between [Ca2+] in the junctional SR and [Ca2+] in the submembrane space (i.e., CajSR − Casub) and by a gating variable [O(t)] describing the transition of RyRs to the open state: jSRCarel = ks × O × (CajSR − Casub), where ks is a constant (the maximum Ca2+ release flux). In turn, the O(t) rate is increased as a nonlinear function of intra-SR [Ca2+] (CajSR) and extra-SR [Ca2+] (Casub). Figure 7 shows simulations of simultaneous changes of Vm (to assess the DD phase), Casub, CajSR, and jSRCarel for basal beating (“Basal” traces), β-AR stimulation (“ISO” traces), and ChR stimulation with 100 nM ACh (“ACh” traces). The results of the analysis of the interplay of these factors to activate the diastolic release are as follows.
Fig. 7.
Model prediction for the mechanisms of spontaneous Ca2+ release in rabbit SANCs and their modulation by β-AR or ChR stimulation. Shown are model simulations of the simultaneous changes in Vm, Casub, CajSR, and Ca2+-release flux (jSRCarel). While the increase in diastolic Ca2+ release is initiated by a rising CajSR, a subsequent strong increase in Ca2+ release and local (within the submembrane space) Ca2+-release synchronization are due an increasing Casub (i.e., Ca2+-induced Ca2+ release).
Item 1.
During very early DD, the Ca2+ transient in the submembrane space is decaying (Casub), but SR loading is increasing in all three cases. Thus, Casub decay “pushes” jSRCarel down, but rising CajSR “pushes” it up. Since Casub decay dominates on its effect on jSRCarel, the release flux is decaying simultaneously with the Casub decay.
Item 2.
The decaying Casub and jSRCarel reach their nadir at approximately the same time (vertical dashed line in Fig. 7). The nadir for Casub is at about the same relatively low level of ∼130 nM (horizontal dashed line in Fig. 7). An interesting model prediction is that the nadir for jSRCarel is not negligible and becomes especially noticeable in the case of isoproterenol treatement [consistent with experimental results demonstrating that early DD is also accelerated by the Ca2+ release during β-AR stimulation (2)]. In other words, the predicted diastolic release has two components: a smaller “persistent” or “tonic” component (evident at the jSRCarel nadir) and a larger “phasic” (i.e., increasing) component, as described below.
Item 3.
Once Casub decay is complete, rising CajSR becomes dominating in its effect on jSRCarel. Thus, the next important model prediction is that the initial, “phasic” diastolic spontaneous Ca2+ release (i.e., Ca2+ release increase) is triggered/governed by the increasing CajSR (i.e., SR Ca2+ load).
Item 4.
While we did not change intrinsic RyR release activation by intra-SR Ca2+ (parameter EC50_SR; see the Supplemental Material for details), the effective release threshold (release begins to increase) is higher for isoproterenol treatment but lower for ACh treatment. This happens because during about the same period of time (before the release nadir, marked by vertical dashed line in Fig. 7) a higher rate of SR refilling with Ca2+ brings CajSR to a higher level (solid horizontal arrows in CajSR panel in Fig. 7). Of note, the effective Ca2+ release threshold is lower than the peak of CajSR (i.e., an “apparent” threshold) because when Ca2+ release is activated, its initial amplitude is relatively small not sufficient to deplete the SR; the Ca2+ level in the SR, in fact, still continues to increase until the release flux and refilling flux equilibrate (at the CajSR peak).
Item 5.
Diastolic Ca2+ release is more synchronized in the case of isoproterenol treatment (more Ca2+ is released per unit time; see the jSRCarel panels in Figs. 3A and 7) but is less synchronized (rising very slowly) in the case of ACh treatment (Figs. 6A and 7). The more release synchronization in the case of isoproterenol is due to a stronger increase in Casub (CajSR is declining during this phase). Thus, while the diastolic release increase is initiated by an increase in CajSR (i.e., by SR Ca2+ refilling; see item 3), the subsequent and greater increase and synchronization of Ca2+ release is due to a rising Casub (i.e., a secondary release, or Ca2+-induced Ca2+ release). An important consequence of the increased synchronization of release is that the release flux achieves a larger amplitude in a shorter time in the case of isoproterenol (i.e., time from the jSRCarel nadir to its maximum, shown by the horizontal arrows in the jSRCarel panel in Fig. 7). Conversely, in the case of ACh treatment, less synchronization of diastolic release results in a smaller release amplitude achieved at a longer time (see the discussion for details).
Item 6.
While the grater increase in Casub during DD is mainly contributed by the SR Ca2+ release into a small volume of submembrane space, it is also contributed (albeit to a lesser degree) by a small Ca2+ influx via subthreshold ICaL activation. During basal state beating, the subthreshold ICaL reaches its maximum of 8.87 pA at −40 mV (see Supplemental Fig. S2), i.e., only ∼19% of the Ca2+-release current (i.e., trans-SR Ca2+ current) at this voltage during DD (45.8 pA; Fig. 3A). In the case of isoproterenol treatment, the relative ICaL contribution becomes smaller (11.8 vs. 115.4 pA, i.e., ∼10%; Fig. 3A). However, in the case of ACh treatment, the predicted relative ICaL contribution substantially increases and becomes comparable with that of jSRCarel (8 vs. 19.1 pA, i.e., ∼42%; Fig. 6A).
Simulation of GPCR Modulation in Cells With Different Basal Parameters
Since the model predictions are sensitive to the input parameters, we tested and confirmed the robustness of our finding of the diastolic Ca2+ release importance in GPCR modulation in two additional simulation series. In these two basal model settings, two major sarcolemmal conductances (gCaL,max and gIf,max, for ICaL and If) were substantially increased (“More If and ICaL” model set) or decreased (“Less If and ICaL” model set) by 25% versus their original values in our primary model [note that the simulated ICaL and If of these two sets were still within experimental range reported by Honjo et al. (19); not shown]. We performed parameter sensitivity analysis to find solutions for the experimental β-AR stimulation effect and also to predict ACh modulation within 0–100 nM (see results in Supplemental Tables 3 and 4). In both additional model parameter sets, the rate regulation via only membrane clock parameter changes was ineffective and substantially less than that when changes in SR Ca2+ cycling were added (i.e., via all mechanisms). In the case of β-AR stimulation, additional changes in Pup were required to reach the experimentally documented 25.8% rate increase. Interestingly, AP firing ceased in simulations with the “Less If and ICaL” model set when [ACh] increased above 43 nM, indicating that ICaL and If are important fail-safe mechanisms for SANC automaticity at higher [ACh] (see the discussion for details).
DISCUSSION
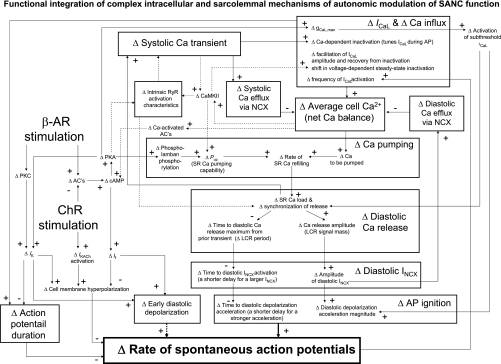
While all prior SANC models (12 models since 1980, according to Ref. 54) have been mainly based on a H-H-type membrane voltage oscillator, the present numerical study revealed that the rate of spontaneous APs in cardiac pacemaker cells is modulated by GPCR signaling via a complex dynamic integration of voltage- and Ca2+-dependent processes within the SANC system (Fig. 8), consistent with experimental results (32, 47, 49, 50, 55) and recent reviews (27, 34). The shifts (marked by Δ) in the balance of system components (which ultimately determine the chronotropic response) are looped and are thus interdependent. In such a complex system, there are no “simple” reactions to GPCR signaling, like a simple activation of “the pacemaker channel” (10) or simple NCX activation by a “Ca2+ oscillator” or “Ca2+ clock” (35, 51). All perturbations, in fact, change the system balance (e.g., cell Ca2+ balance or steady-state DD/AP shape) that directly or indirectly affect all system components. The effective modulation of such a complex system cannot be achieved by perturbing just a single system component (the robust system with redundant mechanisms may compensate the change) but rather requires a modulation of system nodes to orchestrate simultaneous unidirectional shifts in numerous system elements to achieve a true higher (or lower) system balance (rather than a compensatory reaction), as described below.
Fig. 8.
Schematical illustration of our hypothesis of how autonomic modulation impacts on functional integration of numerous interdependent intracellular and sarcolemmal mechanisms to effect changes in the spontaneous AP firing rate. The hypothesis is based on available data in the literature and the numerical modeling of the present study. See the discussion for details.
A Novel Pacemaker Rate Regulation Paradigm via Coupling Factors
A recent experimental study (50) in rabbit SANCs discovered that phospholamban is strongly phosphorylated in the basal state, thus providing a new rate regulation mechanism: gradations in the SR Ca2+ pumping rate that result in gradations of LCR characteristics. The physiological rate increase by the activation of β-ARs is linked to a greater extent of phospholamban phosphorylation (50) resulting in earlier, more frequent, and larger diastolic Ca2+ releases (Figs. 3B and 8). In contrast, the physiological rate decrease by the activation of ChRs is linked to phospholamban dephosphorylation (Fig. 4A), resulting in later, less frequent, and smaller diastolic releases (Figs. 6B and 8) (32). In our basal state model, the capability of SR Ca2+ pumping, Pup, is relatively high [12 vs. 5 mM/s in the Kurata model (24)], simulating experimentally documented basal phospholamban phosphorylation that was not considered in Kurata model. Various degrees of Pup in our model (mimicking the regulatory function of phospholamban) modulate the AP firing rate by coordinated changes of the diastolic Ca2+ release phase and amplitude, which, in turn, modulate late diastolic INCX, in line with aforementioned data in rabbit SANCs (Figs. 3, 6, and 8).
According to our hypothesis (Fig. 8), the SANC AP firing rate is regulated by “enzymatic” coupling factors (36), such as cAMP-mediated, PKA-dependent, and calmodulin kinase II (CaMKII)-dependent protein phosphorylation and by Ca2+, per se, that simultaneously influence voltage- and Ca2+-dependent mechanisms via the following sequence of events: modulation of ICaL and Pup (and also likely RyR activation characteristics; see dashed lines in Fig. 8) by PKA and CaMKII activation via protein phosphorylation → diastolic Ca2+ release phase and amplitude → INCX phase and amplitude → DD acceleration phase and magnitude → AP rate (see results and Fig. 8 for details). These dynamic interactions within the model result in a new steady-state Ca2+ balance (via a feedback from the ΔAP rate to Δfrequency of ICaL activation). In short, these interactions wax in case of β-AR stimulation but wane upon ChR stimulation.
While the diastolic spontaneous release is a novel and critically important factor in autonomic rate regulation, as shown experimentally (32, 49, 50) and in our model simulations (Figs. 3 and 6), specific biophysical mechanisms that regulate spontaneous SR Ca2+ release, per se, have not yet been established for SANCs (or for any other cell type, for that matter). However, our model does explore, in detail, how spontaneous diastolic Ca2+ release emerges and how it is specifically modulated in simulations of β-AR and ChR stimulations (see Detailed Numerical Analysis of How β-AR and ChR Stimulation Modulate Spontaneous SR Ca2+ Release in the Submembrane Compartment and Fig. 7). Our model predicts that the characteristics of diastolic release are regulated by a complex dynamic interplay of SR Ca2+ loading and Ca2+ changes in the submembrane space. In short, while spontaneous, localized (within the submembrane space), diastolic Ca2+ release is initiated by a rising CajSR (i.e., by SR Ca2+ refilling), a subsequent strong increase and synchronization of local Ca2+ releases is due to rising Casub (i.e., a secondary release, or Ca2+-induced Ca2+ release). The earlier and stronger diastolic Ca2+ release in the case of β-AR stimulation but later and smaller release in the case of ChR stimulation in our simulations (Figs. 3 and 6) and previous experimental studies, in fact, can be explained by different degree of diastolic Ca2+-release synchronization (i.e., synchronization of openings of RyRs).
In the case of β-AR stimulation (Fig. 7), spontaneous Ca2+ release can be a threshold event when it involves an “explosive” Ca2+-induced Ca2+-release mechanism in a small volume, like the submembrane space (the SR is separated from sarcolemma by 20 nm in our model). However, the predicted release synchronization of Ca2+ release by RyRs can still be graded by the rate of SR Ca2+ refilling, because this ultimately determines the SR Ca2+ load at which the spontaneous release is initiated as well as the dynamics of the subsequent diastolic release (jSRCarel panel in Fig. 7). Our model predicted that β-AR stimulation leads to a larger loading of the junctional SR (CajSR panel in Fig. 7) and network SR (not shown). An increase in SR Ca2+ loading in the presence of isoproterenol has also been experimentally demonstrated by an increase in caffeine-induced Ca2+ transients in spontaneously beating rabbit SANCs (49).
It is important to note that both increases in intrinsic pump characteristics (Pup) and Ca2+ to be pumped (provided via ICaL) are required to effect the SR Ca2+ load increase in our model (Fig. 8). In fact, a substantial component of Ca2+ influx via L-type Ca2+ channels is likely pumped directly into the SR (22). Thus, additional net Ca2+ to increase the SR Ca2+ load must eventually occur via sarcolemmal Ca2+ influx. During steady-state AP firing during β-AR stimulation, the larger net Ca2+ influx is due to not only ICaL increase per se (i.e., each cycle) but a more frequent ICaL activation at a higher AP rate (over a period of time, say, 1 min; Fig. 8). This greater net Ca2+ influx via ICaL balances the grater Ca2+ efflux from the cell via the larger NCX. When ChRs are stimulated, similar mechanisms, but operating in the opposite direction, produce the SR Ca2+ loading decrease (Fig. 8). Our model predicted that in the presence of 100 nM ACh, SR Ca2+ loading substantially drops (CajSR panel in Fig. 7). This modulation of SR Ca2+ loading is an important model requirement to support larger (or smaller) amplitude diastolic releases, diastolic inward INCX, and DD acceleration (or deceleration) commensurate beating rate changes (Fig. 8).
An important question is whether the documented capacity of phospholamban regulation matches the predicted range of Pup to support physiological rate regulation. According to Eq. 6 (solid curve in Fig. 4A), SR Ca2+ pump inhibition at 100 nM ACh results in a Pup decrease from its basal state value of 12 to 7.58 mM/s. On the other hand, simulation of the effect of β-AR stimulation requires Pup doubling from 12 to 24 mM/s. Thus, GPCR stimulation in our model requires a 3.2-fold range for Pup changes (from 7.58 to 24 mM/s). Experimental studies, however, have shown that Pup modulation via phospholamban phosphorylation is limited only to two to three times [depending on Cai (5)]. Although this is close to our predicted limit, this still indicates that phospholamban/PKA signaling is likely further amplified and/or complemented by some additional factors, such as CaMKII signaling and phosphorylation-dependent effects on RyR activation characteristics, which are not included in our model (dashed lines in Fig. 8; see also Model Limitations and Future Developments below). For example, if β-AR stimulation increases the likelihood for intrinsic activation of RyRs (resulting in a Ca2+ spark increase via local RyR recruitment) in SANCs similar to that in ventricular myocytes (59), this would result in a more synchronized Ca2+ release at a given SR Ca2+ load.
Possible Role of If and “Window” or Subthreshold ICaL in Rhythm Stabilization at Low Rates During ChR Stimulation
While the If amplitude decreases upon ChR stimulation (Fig. 6A), the If contribution becomes more persistent at longer cycle lengths, thus acting as a “stabilizer” of the pacemaker rate [a concept that was first proposed by Noble et al. (41)]. Our interpretation of the role of If as a rate stabilizer (by preventing excessive membrane hyperpolarization; see Fig. 8), especially at low rates and rate transitions, rather than the major rate regulation mechanisms (as previously thought, see the Introduction; via “Δ Early DD” in Fig. 8, dotted arrow) is in line with the recent findings in HCN4 (If channel) knockout mice (16) and mice with the absence of cAMP sensitivity of If (1). Furthermore, a gradual subthreshold diastolic ICaL increase (Fig. 6A and Supplemental Fig. S2), albeit with a slightly smaller amplitude than in the basal state, may also act as another stabilizer of the pacemaker cell system function. During ChR stimulation, ICaL becomes as much as INCX at the end of DD, e.g., 8.1 vs. 8.7 pA at −40 mV (Fig. 6A). Another interesting model finding was that when the diastolic Ca2+ release becomes insufficient to synchronize itself via Ca2+-induced Ca2+ release in the submembrane space, the Ca2+ flux of this persistent subthreshold ICaL becomes a major contributor to the late diastolic Casub elevation and, hence, also indirectly activates late diastolic INCX to support late DD (compare ICaL and jSRCarel panels, both in pA, in Fig. 6A).
Role of IKACh Activation During ChR Stimulation
The comparison of the two dose-response curves (“All mechanisms” and “All mechanisms except IKACh” curves) in Fig. 5 revealed that the effect of IKACh blockade is relatively small at low [ACh]. For example, at 20 nM ACh, the respective rate reductions are 6% versus 7.4%, i.e., an ∼19% difference, consistent with the experimental results that the IKACh contribution becomes evident only at [ACh] > 30 nM (32). At [ACh] = 100 nM, IKACh provides a major contribution to the rate decrease (about one-half of the full effect, 17.5% vs. 40%; Fig. 5), also consistent with the most recent experimental results of ACh-induced beating rate reduction in the presence of IKACh blockade with tertiapin-Q (32). Previous experimental and theoretical studies (4, 6, 9, 20, 32) have shown that the IKACh mechanism dominates in the negative chronotropic effect at higher ACh concentrations (∼1 μM, not studied here).
Comparisons With Other SANC Models
Curiously, despite extensive theoretical studies of cardiac pacemaker function [since 1960 (39)] in numerous numerical SANC models (54), only two model studies (6, 18), to our knowledge, have numerically explored the effect of β-AR stimulation. The 1999 Demir et al. model (6) of the rabbit SANC, however, was based on a previous 1994 Demir et al. model (7) of the SANC. Following an even earlier (1987) modeling study of atrial myocytes by Hilgemann and Noble (17), the Ca2+-release activation in the 1994 model was triggered by membrane depolarization, thereby assuming, by definition, the dominance of membrane mechanisms in cardiac pacemaker function. This type of Ca2+ release, however, was later found to be a key feature of skeletal muscle rather than cardiac muscle (13). Based on the knowledge of SANC ion currents at that time, it was also suggested that the spontaneous SANC firing rate is controlled mainly via cAMP-dependent, hyperpolarization-activated current (If), which regulates the slope of early DD (6). Late DD in many classical SANC models, including the 1994 Demir et al. model, is greatly contributed by T-type Ca2+ current (ICaT) and voltage-gated Na+ current (INa). Both INa and ICaT have low voltage activation thresholds and, therefore, strongly amplified the initial cAMP-sensitive signaling of If-induced early depolarization in the 1999 Demir et al. model (6). However, ICaT was later found to be relatively small and detected only in 20% of rabbit SANCs (38); INa is negligible in primary/central rabbit SANCs (initiating excitations in the sinoatrial node). Furthermore, being cAMP/PKA independent, ICaT cannot control late DD and the pacemaker rate.
Another, more recently proposed mechanism of the β-AR stimulation effect via a nonselective current (Ist) has been investigated in the Kyoto model of guinea pig SANCs (18, 44). However, neither the molecular origin nor specific blockers for Ist have not yet been identified; Ist exhibits many properties of ICaL and INCX and, therefore, might not even be a discrete entity [see more in our recent review (27)]. Therefore, if one excludes INa, ICaT, and Ist as major factors in the autonomic modulation of the AP firing rate of primary SANCs, then changes in If, ICaL, and IKr seem to be insufficient to numerically explain the effect of β-AR stimulation either in our novel model or in previous classical models (open bars in Fig. 2B). Predictions of these classical SANC models, in fact, greatly vary (Fig. 2B). While increases in If and subthreshold ICaL accelerate the beating rate, the ICaL increase during AP actually prolongs the AP duration and, hence, decelerates the beating rate (Fig. 8). The effect of an IKr increase is also dual (Fig. 8): it facilitates the classic “gK decay” pacemaker mechanism (39) (accelerating the beating rate) but simultaneously hyperpolarizes maximum diastolic potential (decelerating the beating rate). Thus, the great variability of predictions in previous sinatrial node models can be explained by their different contributions of these “conflicting” ion channel-related mechanisms. Our simulations showed that the experimentally documented AP rate change is linked to diastolic Ca2+ release (Figs. 3A, 7, and 8). Except for our recent model (36), this new specific mechanism is absent in at least 12 rabbit SANC models developed so far (for a review, see Ref. 54). Thus, the new LCR-induced INCX mechanism coupled to DD acceleration and AP firing rate may be indeed envisioned as the “missing link in the mystery of heart rate regulation” (28) and perhaps explains the paradox of paucity of numerical studies of heart rate regulation by β-ARs.
Cellular mechanisms of AP firing rate downregulation by ChRs have been numerically approached, to our knowledge, in three studies: by Dokos et al. in 1996 (12), by Demir et al. in 1999 (6) (discussed above), and by Zhang et al. in 2002 (58). The latter model simulated the effect of ChR stimulation specifically in central/primary SANCs. The authors assumed that Ca2+ dynamics are not important in these cells and set intracellular Ca2+ to a constant. A recent study (31), however, has shown that Ca2+ cycling is also critical to AP firing of smaller-sized SANCs, which are thought to represent central SANCs (i.e., primary pacemaker cells). Thus, our model simulations here provide the first numerical evidence that Ca2+ cycling is indeed a crucial system component that is required to both the rate increase and decrease via stimulation of β-ARs and ChRs, respectively (Fig. 8).
Model Limitations and Future Developments
Limitation 1.
CaMKII signaling has an important role in SANC function (52, 55), and its well-known properties to amplify and integrate Ca2+ signaling indicate that it may serve as yet another synergistic coupling factor within the SANC pacemaker system (dashed lines to and from “CaMKII” in Fig. 8). However, there is a paucity of experimental data in SANCs that could be used in the numerical modeling of this signaling pathway. Future modeling of SANC studies ought to explore interactions among multiple regulatory or coupling factors, such as Ca2+, CaMKII, and PKA.
Limitation 2.
Basal phosphorylation of RyRs in rabbit SANCs, as assessed by an anti-phosphorylated RyR2 antibody, is substantially higher in rabbit SANCs than in rabbit ventricular cells (50). Also, β-AR stimulation of ventricular myocytes increases the Ca2+ spark amplitude (and Ca2+ current of a single Ca2+ spark) via enhanced recruitment of RyRs (59). These results indicate that the intrinsic characteristics of RyR activation can be modulated in SANCs (dashed lines to and from “RyR” in Fig. 8). However, because of lack biophysical experimental data in SANCs, phosphorylation-dependent changes of RyR were not explored in the present study.
Limitation 3.
Our present model considers only a lumped Ca2+ release signal generated by the junctional SR, whereas, in reality, spontaneous Ca2+ release is produced by multiple stochastic LCRs by local SR fragments (Figs. 3B and 6B). However, the fine ultrastructure of the SR and the detailed distribution of Ca2+ RyRs within SANCs underlying its spontaneous diastolic Ca2+ wavelets are presently unknown and require further experimental studies (for pilot attempts to model stochastic LCRs in rabbit SANC, see Ref. 33). Nonetheless, our present approach is sufficient to reproduce the experimentally documented effects of β-AR and ChR stimulation (Figs. 2, 3, 5, and 6) and does not deny or contradict the idea of stochastic LCRs emerging synchronously during DD. Indeed, while each ion channel has stochastic gating, the synchronized action of the channels' ensemble (e.g., an AP) is closely described by simple H-H-type differential equations. The ensemble of SR Ca2+-release channels (RyRs) also generates a remarkable macroscopic net signal, the late diastolic Ca2+ elevation, observed in the sinoatrial node, a new mechanism of heart rate acceleration via GPCR (23). The novel SANC model described herein reproduces this important macroscopic signal (i.e., an integral of all LCRs within each cycle) and its importance for GPCR modulation (see Figs. 3A and 6A, jSRCarel panel).
Limitation 4.
Our present model does not describe the kinetics of the evolution of the responses to β-AR and ChR stimulation. Additional experimental data that define the transient state kinetics of cAMP, PKA, and CaMKII signaling in spontaneously firing SANCs are required for systems modeling of biochemistry kinetics to simulate the transitions.
Limitation 5.
Data in the literature have indicated that in addition to Cav1.2, Cav1.3 is also present in SANCs and is involved in DD (37). Our model, however, is limited to only one ICaL formulation that integrates both channels. Accurate ICaL description in future SANC models will need to incorporate the respective, specific formulations for the two ICaL types (Cav1.2 and Cav1.3).
GRANTS
This work was supported by the Intramural Research Program of the National Institute on Aging (National Institutes of Health).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Catherine Lloyd and Alan Garny (both from Oxford University) for translating many SANC models, including our original model (36), into CellML format (http://www.cellml.org), which greatly helped to compare predictions of the models (Fig. 2B).
Footnotes
Supplemental Material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Alig J, Marger L, Mesirca P, Ehmke H, Mangoni ME, Isbrandt D. Control of heart rate by cAMP sensitivity of HCN channels. Proc Natl Acad Sci USA 106: 12189–12194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res 99: 979–987, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res 88: 1254–1258, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Boyett MR, Kodama I, Honjo H, Arai A, Suzuki R, Toyama J. Ionic basis of the chronotropic effect of acetylcholine on the rabbit sinoatrial node. Cardiovasc Res 29: 867–878, 1995 [PubMed] [Google Scholar]
- 5.Colyer J, Wang JH. Dependence of cardiac sarcoplasmic reticulum calcium pump activity on the phosphorylation status of phospholamban. J Biol Chem 266: 17486–17493, 1991 [PubMed] [Google Scholar]
- 6.Demir SS, Clark JW, Giles WR. Parasympathetic modulation of sinoatrial node pacemaker activity in rabbit heart: a unifying model. Am J Physiol Heart Circ Physiol 276: H2221–H2244, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Demir SS, Clark JW, Murphey CR, Giles WR. A mathematical model of a rabbit sinoatrial node cell. Am J Physiol Cell Physiol 266: C832–C852, 1994 [DOI] [PubMed] [Google Scholar]
- 8.DiFrancesco D. The contribution of the “pacemaker” current (if) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol 434: 23–40, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiFrancesco D, Ducouret P, Robinson RB. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science 243: 669–671, 1989 [DOI] [PubMed] [Google Scholar]
- 10.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 351: 145–147, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Dokos S, Celler B, Lovell N. Ion currents underlying sinoatrial node pacemaker activity: a new single cell mathematical model. J Theor Biol 181: 245–272, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Dokos S, Celler BG, Lovell NH. Vagal control of sinoatrial rhythm: a mathematical model. J Theor Biol 182: 21–44, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Griffiths H, MacLeod KT. The voltage-sensitive release mechanism of excitation contraction coupling in rabbit cardiac muscle is explained by calcium-induced calcium release. J Gen Physiol 121: 353–373, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagiwara N, Irisawa H, Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol 395: 233–253, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath BM, Terrar DA. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J Physiol 522: 391–402, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. HCN4 provides a “depolarization reserve” and is not required for heart rate acceleration in mice. EMBO J 26: 4423–4432, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilgemann DW, Noble D. Excitation-contraction coupling and extracellular calcium transients in rabbit atrium: reconstruction of basic cellular mechanisms. Proc R Soc Lond B Biol Sci 230: 163–205, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Himeno Y, Sarai N, Matsuoka S, Noma A. Ionic mechanisms underlying the positive chronotropy induced by β1-adrenergic stimulation in guinea pig sinoatrial node cells: a simulation study. J Physiol Sci 58: 53–65, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Honjo H, Boyett MR, Kodama I, Toyama J. Correlation between electrical activity and the size of rabbit sino-atrial node cells. J Physiol 496: 795–808, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honjo H, Kodama I, Zang WJ, Boyett MR. Desensitization to acetylcholine in single sinoatrial node cells isolated from rabbit hearts. Am J Physiol Heart Circ Physiol 263: H1779–H1789, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Huser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol 524: 415–422, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janczewski AM, Lakatta EG. Buffering of calcium influx by sarcoplasmic reticulum during the action potential in guinea-pig ventricular myocytes. J Physiol 471: 343–363, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joung B, Tang L, Maruyama M, Han S, Chen Z, Stucky M, Jones LR, Fishbein MC, Weiss JN, Chen PS, Lin SF. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation 119: 788–796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurata Y, Hisatome I, Imanishi S, Shibamoto T. Dynamical description of sinoatrial node pacemaking: improved mathematical model for primary pacemaker cell. Am J Physiol Heart Circ Physiol 283: H2074–H2101, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Kurata Y, Hisatome I, Imanishi S, Shibamoto T. Roles of L-type Ca2+ and delayed-rectifier K+ currents in sinoatrial node pacemaking: insights from stability and bifurcation analyses of a mathematical model. Am J Physiol Heart Circ Physiol 285: H2804–H2819, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol 47: 157–170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106: 659–673, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakatta EG, Vinogradova TM, Maltsev VA. The missing link in the mystery of normal automaticity of cardiac pacemaker cells. Ann NY Acad Sci 1123: 41–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei M, Brown HF, Terrar DA. Modulation of delayed rectifier potassium current, iK, by isoprenaline in rabbit isolated pacemaker cells. Exp Physiol 85: 27–35, 2000 [PubMed] [Google Scholar]
- 30.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res 74: 1071–1096, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, Spurgeon HA, Sollott SJ, Lakatta EG. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res 100: 1723–17312007 [DOI] [PubMed] [Google Scholar]
- 32.Lyashkov AE, Vinogradova TM, Zahanich I, Li Y, Younes A, Nuss HB, Spurgeon HA, Maltsev VA, Lakatta EG. Cholinergic receptor signaling modulates spontaneous firing of sinoatrial nodal cells via integrated effects on PKA-dependent Ca2+ cycling and IKACh. Am J Physiol Heart Circ Physiol 297: H949–H959, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maltsev AV, Maltsev VA, Mikheev M, Maltseva LA, Sirenko SG, Lakatta EG, Stern MD. Emergence of local Ca oscillators in cardiac pacemaker cells: 2D Ca dynamics measurements, an analytical theory, and complex systems numerical modeling (Abstract). Biophys J 98, Suppl 1: 21a [Google Scholar]
- 34.Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res 77: 274–284, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Maltsev VA, Lakatta EG. Normal heart rhythm is initiated and regulated by an intracellular calcium clock within pacemaker cells. Heart Lung Circ 16: 335–348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol 296: H594–H615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci USA 100: 5543–5548, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangoni ME, Fontanaud P, Noble PJ, Noble D, Benkemoun H, Nargeot J, Richard S. Facilitation of the L-type calcium current in rabbit sino-atrial cells: effect on cardiac automaticity. Cardiovasc Res 48: 375–392, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Noble D. Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations. Nature 188: 495–497, 1960 [DOI] [PubMed] [Google Scholar]
- 40.Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol 353: 1–50, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble D, Denyer JC, Brown HF, DiFrancesco D. Reciprocal role of the inward currents ib, Na and if in controlling and stabilizing pacemaker frequency of rabbit sino-atrial node cells. Proc Biol Sci 250: 199–207, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Noma A, Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflügers Arch 377: 193–200, 1978 [DOI] [PubMed] [Google Scholar]
- 43.Petit-Jacques J, Bois P, Bescond J, Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflügers Arch 423: 21–27, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Sarai N, Matsuoka S, Kuratomi S, Ono K, Noma A. Role of individual ionic current systems in the SA node hypothesized by a model study. Jpn J Physiol 53: 125–134, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J 87: 3351–3371, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stern MD, Song LS, Cheng H, Sham JS, Yang HT, Boheler KR, Rios E. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J Gen Physiol 113: 469–489, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Borren MM, Verkerk AO, Wilders R, Hajji N, Zegers JG, Bourier J, Tan HL, Verheijck EE, Peters SL, Alewijnse AE, Ravesloot JH. Effects of muscarinic receptor stimulation on Ca2+ transient, cAMP production and pacemaker frequency of rabbit sinoatrial node cells. Basic Res Cardiol 105: 73–87, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verheijck EE, van Ginneken AC, Wilders R, Bouman LN. Contribution of L-type Ca2+ current to electrical activity in sinoatrial nodal myocytes of rabbits. Am J Physiol Heart Circ Physiol 276: H1064–H1077, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Vinogradova TM, Bogdanov KY, Lakatta EG. β-Adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res 90: 73–79, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Vinogradova TM, Maltsev VA, Bogdanov KY, Lyashkov AE, Lakatta EG. Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann NY Acad Sci 1047: 138–156, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, Xiao RP. Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase II activation. Circ Res 87: 760–767, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res 94: 802–809, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Wilders R. Computer modelling of the sinoatrial node. Med Biol Eng Comput 45: 189–207, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, Wehrens XH, Mohler PJ, Song LS, Anderson ME. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci USA 106: 5972–5977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaza A, Robinson RB, DiFrancesco D. Basal responses of the L-type Ca2+ and hyperpolarization-activated currents to autonomic agonists in the rabbit sino-atrial node. J Physiol 491: 347–355, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, Holden AV, Kodama I, Honjo H, Lei M, Varghese T, Boyett MR. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am J Physiol Heart Circ Physiol 279: H397–H421, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, Holden AV, Noble D, Boyett MR. Analysis of the chronotropic effect of acetylcholine on sinoatrial node cells. J Cardiovasc Electrophysiol 13: 465–474, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Zhou P, Zhao YT, Guo YB, Xu SM, Bai SH, Lakatta EG, Cheng H, Hao XM, Wang SQ. β-Sdrenergic signaling accelerates and synchronizes cardiac ryanodine receptor response to a single L-type Ca2+ channel. Proc Natl Acad Sci USA 106: 18028–18033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.