Abstract
CXC-chemokine ligand 10 (CXCL10) inhibits angiogenesis and attracts activated T lymphocytes. Abnormal angiogenesis and lymphocytic infiltration participate in the pathobiology of pulmonary arterial hypertension (PAH). We hypothesized that serum CXCL10 is elevated in idiopathic PAH and that it is associated with clinical outcomes. This was a cohort study that included 40 idiopathic PAH patients (age = 44 ± 14 years, 37 females) and 22 healthy controls (age = 35 ± 6 years, 18 females). It took place at the Pulmonary Vascular Program at the Cleveland Clinic. Serum CXCL10 levels were measured by an enzyme-linked immunosorbent assay. A cutoff value of CXCL10 for best distinguishing alive and dead patients was obtained from a receiver operating characteristic curve (ROC). Survival and time to clinical worsening curves according to the appropriate CXCL10 level were derived by the Kaplan–Meier method and compared by means of the log-rank test. The prognostic value of CXCL10 and of other variables of interest was tested by Cox proportional hazards regression analysis. Serum CXCL10 levels were elevated in PAH subjects compared to controls [CXCL10 pg/ml (mean ± SEM) for PAH: 306 ± 73, and for controls: 92 ± 10; p < 0.0001]. CXCL10 levels higher than 111 pg/ml discriminated survivors from nonsurvivors with a sensitivity of 81% and a specificity of 75% (area under the ROC curve = 0.74). After a mean follow-up of 23.5 ± 13.5 months since the day of venous sampling, higher CXCL10 levels were associated with improved survival (hazard ratio for mortality = 0.10, 95% confidence interval = 0.01–0.97; p = 0.01). Serum CXCL10 is elevated in PAH and this is associated with improved survival.
Keywords: Pulmonary hypertension, Prognosis, Biomarker, Chemokines
Introduction
While the understanding of the imbalance between vasodilators and vasoconstrictors in pulmonary arterial hypertension (PAH) has led to the currently available targeted therapies, PAH remains a relentlessly progressive incurable disease. Progressive pulmonary vascular remodeling, characterized by smooth muscle cell proliferation, inflammatory cell infiltration, and angiogenic endothelial cell proliferation, is the hallmark of severe PAH [1, 2]. The inciting triggers for these changes as well as their regulatory mechanisms remain incompletely understood. These changes lead to luminal obliteration which in turn causes a progressive increase in pulmonary vascular resistance, right ventricular failure, and premature death.
Chemokines are polypeptides that exert various functions in health and disease [3], with leukocyte chemotaxis and directed cell migration being their common properties [4]. They mediate their effects through interaction with cell membrane-bound G-coupled receptors [4]. There are two main families of chemokines, defined by the relative position of the cysteine residues in the ligands, the CXC and CC families, where C is a cysteine residue and X is another amino acid [5]. CXC chemokines can be further divided into two groups according to the presence or absence of the sequence glutamic acid-leucine-arginine (ELR) near the N terminal. ELR + CXC chemokines are chemotactic for neutrophils and promote angiogenesis, whereas those lacking this sequence attract lymphocytes and inhibit angiogenesis [3, 6]. CXCL10 (formerly known as γ-interferon-induced protein 10 [IP-10]) belongs to the latter group. CXCL10 attracts activated T lymphocytes, mainly of the T helper 1 subgroup [6, 7], and has also been shown to inhibit angiogenesis [8–10]. Given that inflammatory cell infiltration and abnormal angiogenesis are present in PAH, we hypothesize that CXCL10 plays a role in the pathobiology of PAH and that its measurement could provide prognostic information. CXCL10 has not been previously reported in PAH.
Materials and Methods
Study Population and Clinical Outcomes
Patients were recruited from the pulmonary vascular program at the Cleveland Clinic. We enrolled subjects with idiopathic and heritable PAH as defined by the updated clinical classification of pulmonary hypertension from Dana Point 2008 [11]. Other forms of pulmonary hypertension were excluded by a complete workup, as recommended by guidelines [12]. Other exclusion criteria included a confirmed or suspected infection and unstable coronary artery disease. As part of our routine clinical practice, PAH patients undergo a thorough clinical characterization to assess disease severity and proper therapy. Parameters routinely obtained include an assessment of the patient’s exercise capacity by the World Health Organization (WHO) functional classification and the 6-min walk test; transthoracic Doppler echocardiography for signs of right ventricular (RV) dysfunction, e.g., right atrial and ventricular size, RV systolic dysfunction, tricuspid regurgitation, and pericardial effusion; B-type natriuretic peptide (BNP) levels; and pulmonary hemodynamics including right atrial pressure, cardiac index, pulmonary vascular resistance, and mean pulmonary artery pressure. These data were recorded at the time of venous sampling. Healthy volunteers were used as controls.
Clinical outcomes of interest included hospitalization for worsening PAH, lung transplantation, and all-cause mortality since the date of blood sampling. These data were recorded retrospectively by review of the medical record for 18 subjects who had had their serum samples collected and stored in our laboratory. For the remaining 22 patients, data were collected in a prospective fashion. Analysis of all samples was performed in one stage only. Even for the 22 patients whose clinical data were collected prospectively, the venous sample was processed and stored until measurement of serum CXCL10. The study was approved by the Institutional Review Board at the Cleveland Clinic and written informed consent was obtained from all subjects.
Measurement of Serum CXCL10 Levels
After informed consent was obtained, peripheral venous blood was drawn into a serum separator tube and allowed to clot for 30 min before centrifugation for 15 min at 1,000g. Serum samples were stored at −80°C until analyzed. Serum CXCL10 levels were measured by enzyme-linked immunosorbent assay (ELISA) (R & D Systems, Minneapolis, MN, catalog No. DIP100) following the manufacturer’s recommendations. This ELISA has a minimum detection limit of 0.41–4.46 pg/ml and a within-run precision of 3.0–4.6%.
Statistical Analysis
Data are presented as proportions, mean ± standard deviation (SD), and mean ± standard error of the mean (SEM) as appropriate. We used the Wilcoxon rank sum test for the comparison of serum CXCL10 levels between patients and controls, and the Spearman rank test to assess for correlations with variables of interest. For the purpose of identifying a cutoff value of CXCL10 for best distinguishing alive and dead subjects, the value which maximized sensitivity and specificity was obtained from a receiver operating characteristic curve (ROC). Survival and time to clinical worsening curves according to the appropriate CXCL10 level were derived by the Kaplan–Meier method and compared by means of the log-rank test. The prognostic value of CXCL10 and of other variables of interest was tested by univariate Cox proportional hazards regression analysis. With the use of multivariate models, the prognostic power of serum CXCL10 was assessed while adjusting for other predictors that were found significant in univariate analysis. Clinical worsening was defined as a composite end point of death, lung transplantation, and hospitalization due to worsening PAH. Statistical analyses were performed using JMP 8.0 (SAS Institute Inc., Cary, NC, USA) and R version 2.8.1 [13].
Results
Study Population and Serum CXCL10 Levels
We studied 40 patients with idiopathic PAH and 22 healthy volunteers. Baseline demographic, clinical, and hemodynamic features are given in Table 1. As shown in Fig. 1, serum CXCL10 levels were markedly elevated in PAH subjects compared to controls. At the time of venous sampling, 9 patients (22.5%) were not receiving PAH-targeted therapies, 24 were on parenteral prostanoids (60%), 4 were on sildenafil (10%), and 3 were on bosentan (7.5%). CXCL10 levels were similar in both naive patients and those on PAH-targeted therapies (292 ± 61 and 310 ± 93 pg/ml, respectively, p = 0.23). While patients receiving parenteral prostanoids had higher CXCL10 levels than the rest of the cohort, the difference was not statistically significant and it was driven by two outliers (351 ± 119 and 238 ± 39 pg/ml, respectively, p = 0.5).
Table 1.
Baseline demographic and clinical characteristics
| Patients (n = 40) | |
|---|---|
| Age | 44 ± 14 |
| Females | 37 (92.5) |
| Functional class | |
| I–II | 17 (42.5) |
| III–IV | 23 (57.5) |
| 6MWD (m)a | 397 ± 130 |
| BNP (pg/ml) | 140 ± 218 |
| RAP (mmHg) | 10 ± 6 |
| mPAP (mmHg) | 55 ± 13 |
| CI (l/min/m2) | 2.33 ± 1.04 |
| PVR (Wood units) | 11.9 ± 5.5 |
Data presented as number (%) and mean ± SD
n = 39, 1 patient unable to perform test
6MWD 6-min walk distance, BNP B-type natriuretic peptide, CI cardiac index, mPAP mean pulmonary artery pressure, PVR pulmonary vascular resistance, RAP mean right atrial pressure
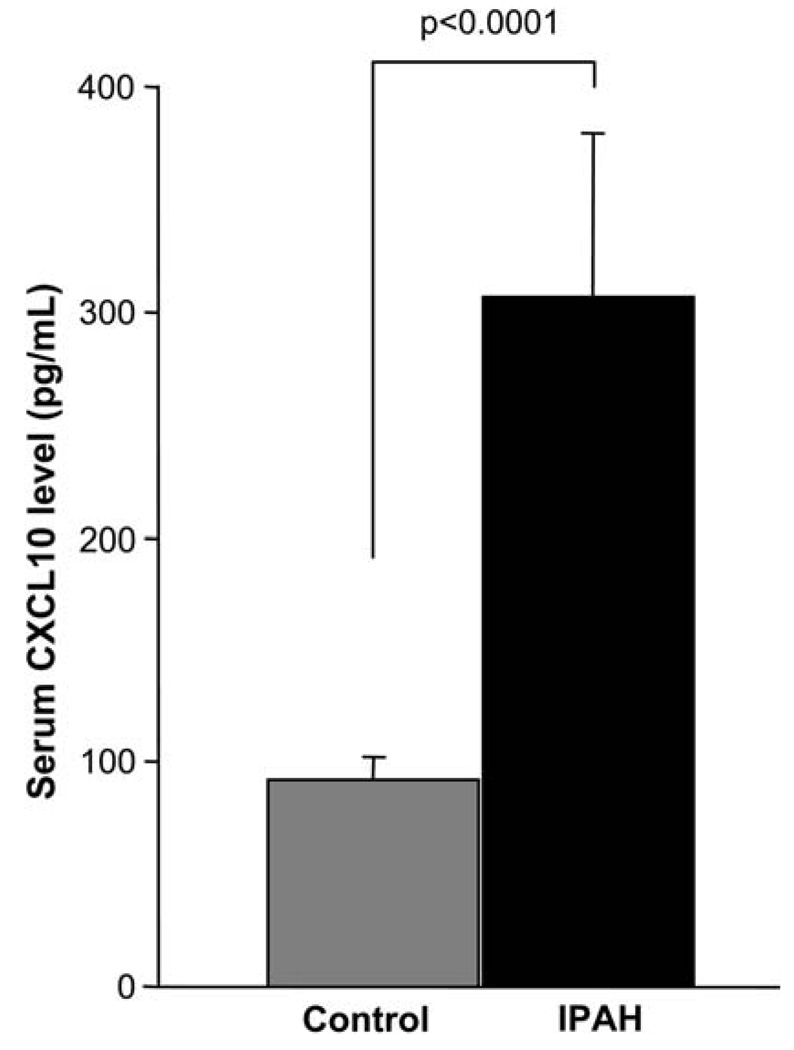
Fig. 1.
Serum CXCL10 levels in IPAH versus controls. Each bar shows the mean ± SEM. IPAH patients have significantly higher serum CXCL10 levels than controls (CXCL10 pg/ml, mean ± SEM: PAH, 306 ± 73; controls, 92 ± 10; p < 0.0001)
Clinical Outcomes
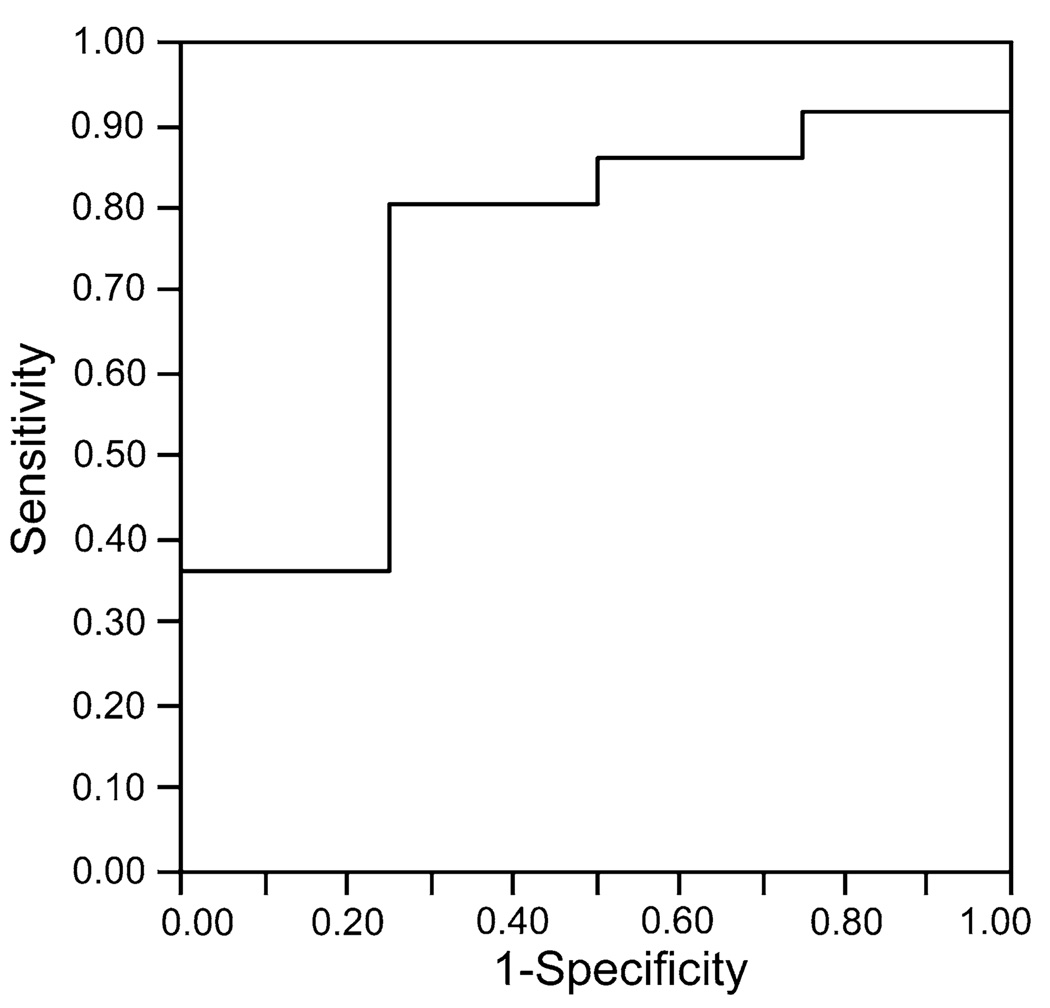
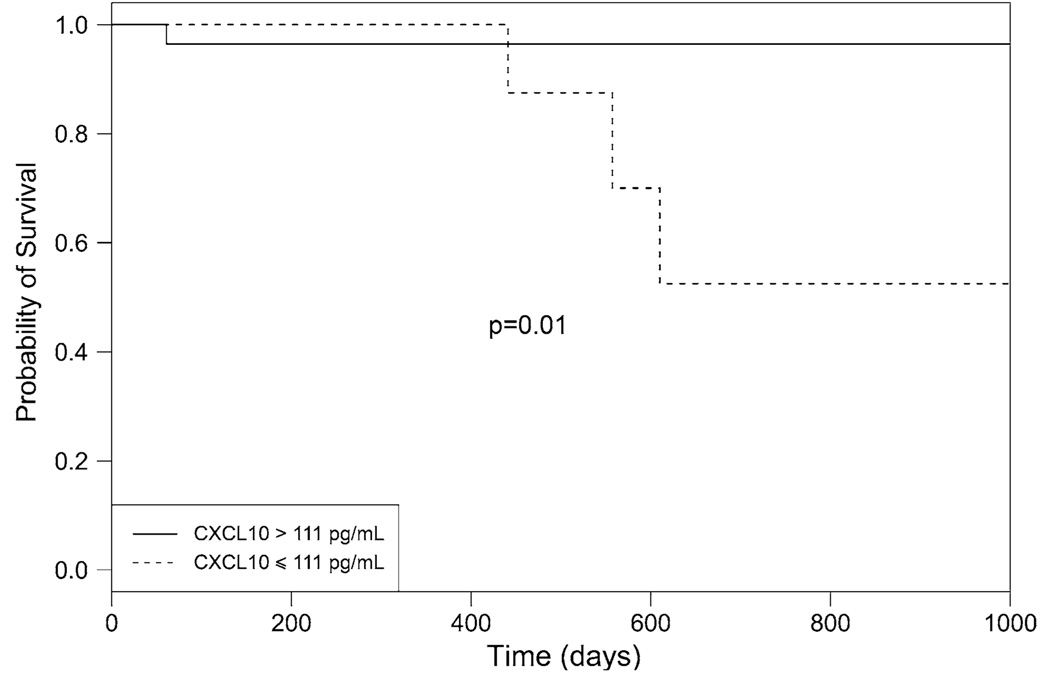
ROC analysis showed that CXCL10 levels higher than 111 pg/ml discriminated survivors from nonsurvivors with a sensitivity of 81% and a specificity of 75% (area under the curve = 0.74, Fig. 2). Mean follow-up was 23.5 ± 13.5 months (range = 1.9–48.7 months) since the day of venous sampling. PAH patients with CXCL10 levels above 111 pg/ml had a higher probability of survival than patients with lower levels (hazard ratio [HR] for mortality = 0.10, 95% confidence interval [CI] = 0.01–0.97, p = 0.01) (Fig. 3). By comparison, ROC analysis showed that BNP levels had no predictive ability in our patient population (area under ROC = 0.46). Survival analysis confirmed that BNP had no association with mortality (HR = 1.20, 95% CI = 0.11–13.28, p = 0.88). The only other variable predictive of mortality was the distance walked in 6 min. ROC analysis identified a cutoff of 384 m at and above which no patient died, compared to four deaths in the group of patients with baseline distance below 384 m (log-rank test 0.04, not able to calculate HR as no events in one group). When adjusted for the baseline 6-min walk distance, serum CXCL10 levels remained predictive of mortality (HR = 0.11, 95% CI = 0.01–1.04, p = 0.05). No variable was predictive of clinical worsening.
Fig. 2.
Receiver operating characteristics curve. CXCL10 >111 pg/ml discriminated survivors from nonsurvivors with a sensitivity of 81% and a specificity of 75% (area under the curve = 0.74)
Fig. 3.
Survival according to CXCL10 levels. One of 30 patients (3.3%) with CXCL10 levels >111 pg/ml died, compared to three out ten (30%) of those with levels ≤111 pg/ml (hazard ratio for mortality, 0.10; 95% CI = 0.01–0.97, p = 0.01)
CXCL10 and PAH Severity
Table 2 depicts the clinical characteristics of the patient cohort divided by the 111-pg/ml cutoff level. Subjects with higher CXCL10 concentrations had a tendency to be in WHO functional classes III and IV and to have pericardial effusion less frequently, but these differences did not reach statistical significance. Other measures of functional capacity, BNP levels, and hemodynamic parameters were similar between the two groups.
Table 2.
Clinical characteristics according to CXCL10 serum levels
| CXCL10 ≤ 111 pg/ml (n = 11) | CXCL10 > 111 pg/ml (n = 29) | p | |
|---|---|---|---|
| Age (years) | 42 ± 15 | 45 ± 13 | 0.61 |
| Females | 10 (91) | 27 (93) | 1 |
| WHO class III–IV | 4 (36) | 19 (66) | 0.15 |
| 6MWD (m) | 421 ± 102 | 379 ± 128 | 0.42 |
| Pericardial effusion | 4 (36) | 6 (21) | 0.42 |
| BNP (pg/ml) | 136 ± 242 | 142 ± 214 | 0.48 |
| RAP (mmHg) | 11 ±6 | 10 ± 6 | 0.87 |
| mPAP (mmHg) | 55 ± 15 | 54 ± 13 | 1 |
| CI (l/min/m2) | 2.51 ± 1.12 | 2.26 ± 1.02 | 0.4 |
| PVR (Wood units) | 11.05 ± 5.93 | 12.24 ± 5.47 | 0.47 |
6MWD 6-min walk distance, BNP B-type natriuretic peptide, CI cardiac index, mPAP mean pulmonary artery pressure, PVR pulmonary vascular resistance, RAP mean right atrial pressure, WHO World Health Organization
Data presented as mean ± SD and number (%)
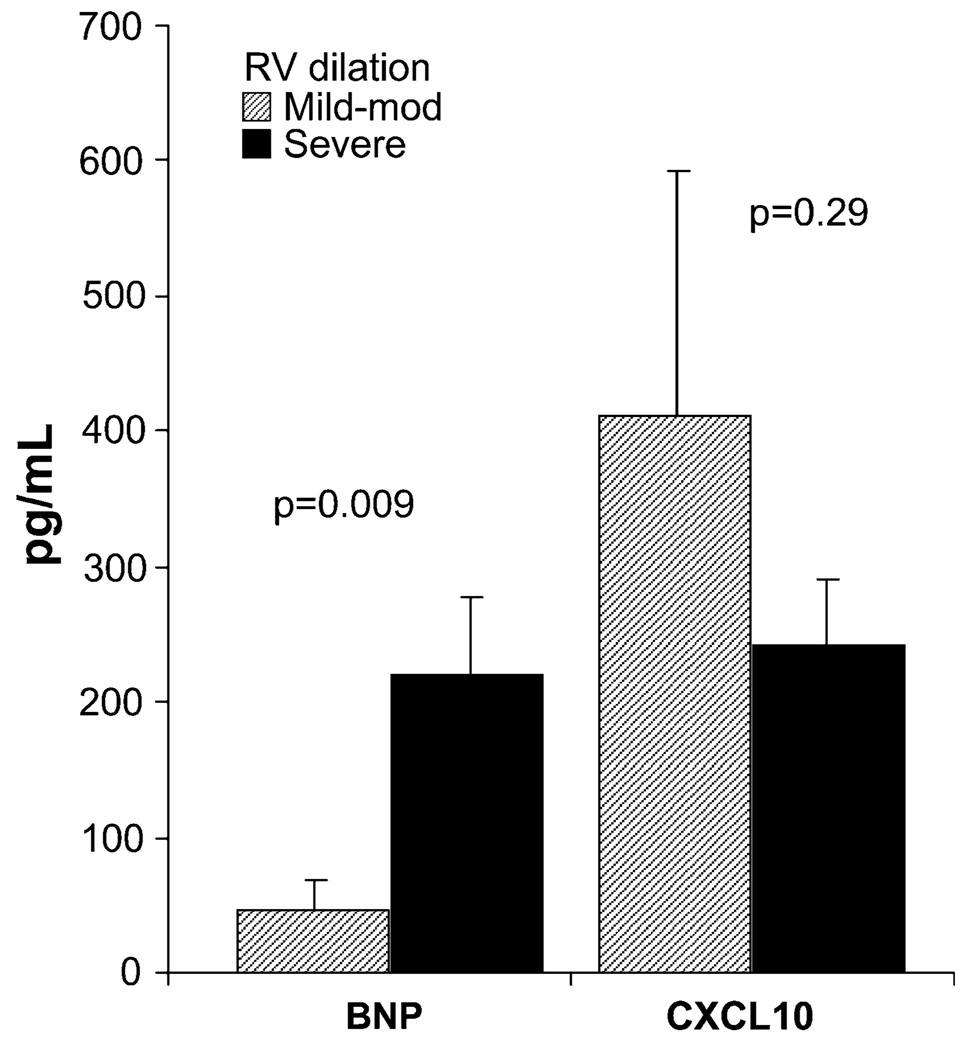
CXCL10 levels did not have any significant correlation with standard parameters of PAH severity (Table 3). There was no difference in CXCL10 levels according to the degree of RV dilation assessed by Doppler echocardiography (Fig. 4) or the functional class (data not shown). In comparison, BNP had significant associations with the distance walked in 6 min, right atrial pressure, mixed venous oxygen saturation (Table 3), and right ventricular dilation (Fig. 4).
Table 3.
Correlations between B-type natriuretic peptide and CXCL10 and standard parameters of PAH severity
| BNP (pg/ml) | CXCL10 (pg/ml) | |
|---|---|---|
| 6MWD (m) | −0.58 (<0.001) | −0.18 (0.30) |
| mPAP (mmHg) | 0.26 (0.10) | 0.01 (0.96) |
| RAP (mmHg) | 0.49 (0.001) | 0.05 (0.75) |
| CI (l/min/m2) | −0.31 (0.07) | 0.03 (0.84) |
| PVR (Wood units) | 0.15 (0.36) | 0.07 (0.68) |
| MVO2 (%) | −0.48 (0.01) | −0.03 (0.86) |
| BNP (pg/ml) | 0.22 (0.18) |
Data presented as Spearman correlation coefficient (p value)
6MWD 6-min walk distance, BNP B-type natriuretic peptide, CI cardiac index, mPAP mean pulmonary artery pressure, MVO2 mixed venous oxygen saturation, PVR pulmonary vascular resistance, RAP mean right atrial pressure
Fig. 4.
BNP and CXCL10 levels according to the severity of right ventricular dilation. Each bar shows the mean ± SEM. BNP was higher with more severe RV dilation (mild to moderate RV dilation, 47 ± 21 pg/ml; severe RV dilation, 219 ± 57 pg/ml, p = 0.009). In comparison, serum CXCL10 levels were not different (mild to moderate RV dilation, 412 ± 180 pg/ml; severe RV dilation, 243 ± 49 pg/ml, p = 0.33). RV right ventricular
Discussion
The main findings of our study are that CXCL10 is elevated in the serum of idiopathic PAH patients and that this elevation is associated with better survival. Remarkably, despite its association with mortality, CXCL10 did not correlate with any of the usual parameters of PAH severity.
Three chemokines have been described as playing a role in PAH: CCL2 (previously known as monocyte chemoattractant protein-1 [MCP-1]) [14, 15], CCL5 (formerly Regulated upon Activation, Normal T-cell Expressed, and Secreted [RANTES] [16], and CX3C or fractalkine [17]. These chemokines are elevated in blood [14, 15, 17] and lungs [15–17] of PAH patients, but they have never been associated with clinical outcomes. CXCL10 is constitutively expressed in the thymus, spleen, and lymph nodes, but its production is highly induced by interferons—particularly γinterferon—in a variety of cells, including monocytes, keratinocytes, endothelial cells, smooth muscle cells, and fibroblasts [18, 19]. CXCL10 interacts with the receptor CXCR3, which is expressed by T cells (especially T helper 1), B cells, natural killer cells, mesangial cells, smooth muscle cells, and endothelial cells [3, 7]. CXCL10 has been implicated in atherosclerosis [20], cardiac allograft rejection [21], sarcoidosis [22], pulmonary fibrosis [23], and chronic obstructive pulmonary disease [24], among various conditions. To the best of our knowledge, CXCL10 has not been previously reported in PAH.
Several blood markers have been investigated in patients with PAH [25], including uric acid [26, 27], von Willebrand factor [28, 29], and natriuretic peptides [30, 31]. In general, these markers are elevated in blood and correlate with exercise capacity and hemodynamics, and their elevation is associated with higher mortality. Our results are unique in that we report the first biomarker that when elevated in the peripheral blood, carries a good prognosis. In the case of natriuretic peptides, the biomarkers most commonly used in clinical practice [25], patients on PAH therapy with high BNP levels (>180 pg/ml) have very poor survival [30]. It is possible that these markers, while effectively reflecting worsening right ventricular failure, provide prognostic information only when the disease is too advanced. Most of our patients were on PAH-targeted therapies, had BNP levels below 180 pg/ml, and had relatively preserved right ventricular function (Table 1). It is interesting that in this population, serum CXCL10 levels were predictive of mortality. Furthermore, serum CXCL10 levels had no correlations with standard prognostic indicators in PAH (Table 3). BNP had significant correlations with several of these parameters in our population, but it had no associations with clinical outcomes.
What may be the mechanism underlying the better outcomes seen with higher serum CXCL10 levels? A recent study suggests that intact T-cell activity is needed to prevent severe pulmonary hypertension and remodeling [32]. In this context, CXCL10 is notable for attracting activated T lymphocytes via its interaction with the receptor CXCR3 [6, 7]. Another factor contributing to vascular remodeling in PAH is exuberant angioproliferation [1, 33, 34]. CXCL10 inhibits angiogenesis [8] and may represent a counterregulatory response to the increase in new vessel formation seen in PAH. Thus, increased CXCL10 activity, through the recruitment of T cells and the inhibition of angiogenesis, could lead to decreased vascular remodeling and better outcomes in idiopathic PAH, as suggested by our data. Under circumstances leading to relatively decreased CXCL10 production, this could in turn lead to decreased T-cell activity, worsened angioproliferation, and shortened survival. This remains speculative.
The main limitation of this study is the relatively small sample size with a small number of clinical events. This could account for the lack of association between BNP and prognosis, as natriuretic peptides have been found to have prognostic implications [30, 31]. This also limits the amount of feasible statistical adjustment for potential confounders. A sizable proportion of patients were on prostacyclin therapy, and it is not clear what impact, if any, these compounds have on CXCL10 serum levels. In a recent study, a selective prostacyclin receptor agonist suppressed interferon-γ-induced CXCL10 release by human airway epithelial cells in vitro [35]. There are no studies in pulmonary vascular cells, and our data do not establish the source of CXCL10 in these patients.
In summary, we report elevated serum CXCL10 in idiopathic PAH and that higher CXCL10 levels are associated with improved survival. Further studies are needed to confirm the prognostic utility of serum CXCL10 measurement and to clarify the mechanisms behind CXCL10 production and regulation in patients affected by idiopathic PAH. CXCL10 appears to be a new promising noninvasive biomarker for this deadly disease.
Acknowledgment
This work was supported by the National Institutes of Health (grant NIH HL68863).
Contributor Information
Gustavo A. Heresi, Email: heresig@ccf.org, Pulmonary and Critical Care Medicine, Respiratory Institute, Cleveland Clinic, 9500 Euclid Avenue A90, Cleveland, OH 44195, USA.
Metin Aytekin, Email: aytekim@ccf.org, Pathobiology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA.
Jennie Newman, Email: newmanj3@ccf.org, Pulmonary and Critical Care Medicine, Respiratory Institute, Cleveland Clinic, 9500 Euclid Avenue A90, Cleveland, OH 44195, USA.
Raed A. Dweik, Email: dweikr@ccf.org, Pulmonary and Critical Care Medicine, Respiratory Institute, Cleveland Clinic, 9500 Euclid Avenue A90, Cleveland, OH 44195, USA.
References
- 1.Voelkel NF, Douglas IS, Nicolls M. Angiogenesis in chronic lung disease. Chest. 2007;131:874–879. doi: 10.1378/chest.06-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:558–564. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 4.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 5.Bacon K, Baggiolini M, Broxmeyer H, Horuk R, Lindley I, Mantovani A, Maysushima K, Murphy P, Nomiyama H, Oppenheim J, Rot A, Schall T, Tsang M, Thorpe R, Van Damme J, Wadhwa M, Yoshie O, Zlotnik A, Zoon K. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- 6.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 7.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210:51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 10.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 11.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, McGregor K, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas P, Widimsky P, Al Attar N, Andreotti F, Aschermann M, Asteggiano R, Benza R, Berger R, Bonnet D, Delcroix M, Howard L, Kitsiou AN, Lang I, Maggioni A, Nielsen-Kudsk JE, Park M, Perrone-Filardi P, Price S, Domenech MT, Vonk-Noordegraaf A, Zamorano JL. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 13.R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. ISBN 3-900051-07-0 http://www.R-project.org. [Google Scholar]
- 14.Itoh T, Nagaya N, Ishibashi-Ueda H, Kyotani S, Oya H, Sakamaki F, Kimura H, Nakanishi N. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology. 2006;11:158–163. doi: 10.1111/j.1440-1843.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, Dartevelle P, Simonneau G, Adnot S, Eddahibi S. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176:1041–1047. doi: 10.1164/rccm.200610-1559OC. [DOI] [PubMed] [Google Scholar]
- 16.Dorfmuller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, Coulomb-Lhermine A, Marfaing-Koka A, Simonneau G, Emilie D, Humbert M. Chemokine RAN-TES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:534–539. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 17.Balabanian K, Foussat A, Dorfmuller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, Marfaing-Koka A, Krzysiek R, Rimaniol AC, Simonneau G, Emilie D, Humbert M. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:1419–1425. doi: 10.1164/rccm.2106007. [DOI] [PubMed] [Google Scholar]
- 18.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 20.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, Gerszten RE. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 21.Melter M, Exeni A, Reinders ME, Fang JC, McMahon G, Ganz P, Hancock WW, Briscoe DM. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–2564. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 22.Agostini C, Cassatella M, Zambello R, Trentin L, Gasperini S, Perin A, Piazza F, Siviero M, Facco M, Dziejman M, Chilosi M, Qin S, Luster AD, Semenzato G. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161:6413–6420. [PubMed] [Google Scholar]
- 23.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, Luster AD. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- 24.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, Sinigaglia F, Fabbri LM. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 25.Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J. 2008;32:503–512. doi: 10.1183/09031936.00160307. [DOI] [PubMed] [Google Scholar]
- 26.Nagaya N, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Nakanishi N, Yamagishi M, Kunieda T, Miyatake K. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:487–492. doi: 10.1164/ajrccm.160.2.9812078. [DOI] [PubMed] [Google Scholar]
- 27.Bendayan D, Shitrit D, Ygla M, Huerta M, Fink G, Kramer MR. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Respir Med. 2003;97:130–133. doi: 10.1053/rmed.2003.1440. [DOI] [PubMed] [Google Scholar]
- 28.Lopes AA, Maeda NY, Bydlowski SP. Abnormalities in circulating von Willebrand factor and survival in pulmonary hypertension. Am J Med. 1998;105:21–26. doi: 10.1016/s0002-9343(98)00138-7. [DOI] [PubMed] [Google Scholar]
- 29.Kawut SM, Horn EM, Berekashvili KK, Widlitz AC, Rosenzweig EB, Barst RJ. von Willebrand factor independently predicts long-term survival in patients with pulmonary arterial hypertension. Chest. 2005;128:2355–2362. doi: 10.1378/chest.128.4.2355. [DOI] [PubMed] [Google Scholar]
- 30.Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, Miyatake K, Kangawa K. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 31.Leuchte HH, Holzapfel M, Baumgartner RA, Neurohr C, Vogeser M, Behr J. Characterization of brain natriuretic peptide in long-term follow-up of pulmonary arterial hypertension. Chest. 2005;128:2368–2374. doi: 10.1378/chest.128.4.2368. [DOI] [PubMed] [Google Scholar]
- 32.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med. 2007;175:1280–1289. doi: 10.1164/rccm.200608-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 34.Masri FA, Anand-Apte B, Vasanji A, Xu W, Goggans T, Drazba J, Erzurum SC. Definitive evidence of fundamental and inherent alteration in the phenotype of primary pulmonary hypertension endothelial cells in angiogenesis. Chest. 2005;128:571S. doi: 10.1378/chest.128.6_suppl.571S. [DOI] [PubMed] [Google Scholar]
- 35.Ayer LM, Wilson SM, Traves SL, Proud D, Giembycz MA. 4,5-Dihydro-1H-imidazol-2-yl)-[4-(4-isopropoxy-benzyl)-phenyl]-amine (RO1138452) is a selective, pseudo-irreversible orthosteric antagonist at the prostacyclin (IP)-receptor expressed by human airway epithelial cells: IP-receptor-mediated inhibition of CXCL9 and CXCL10 release. J Pharmacol Exp Ther. 2008;324:815–826. doi: 10.1124/jpet.107.129312. [DOI] [PubMed] [Google Scholar]