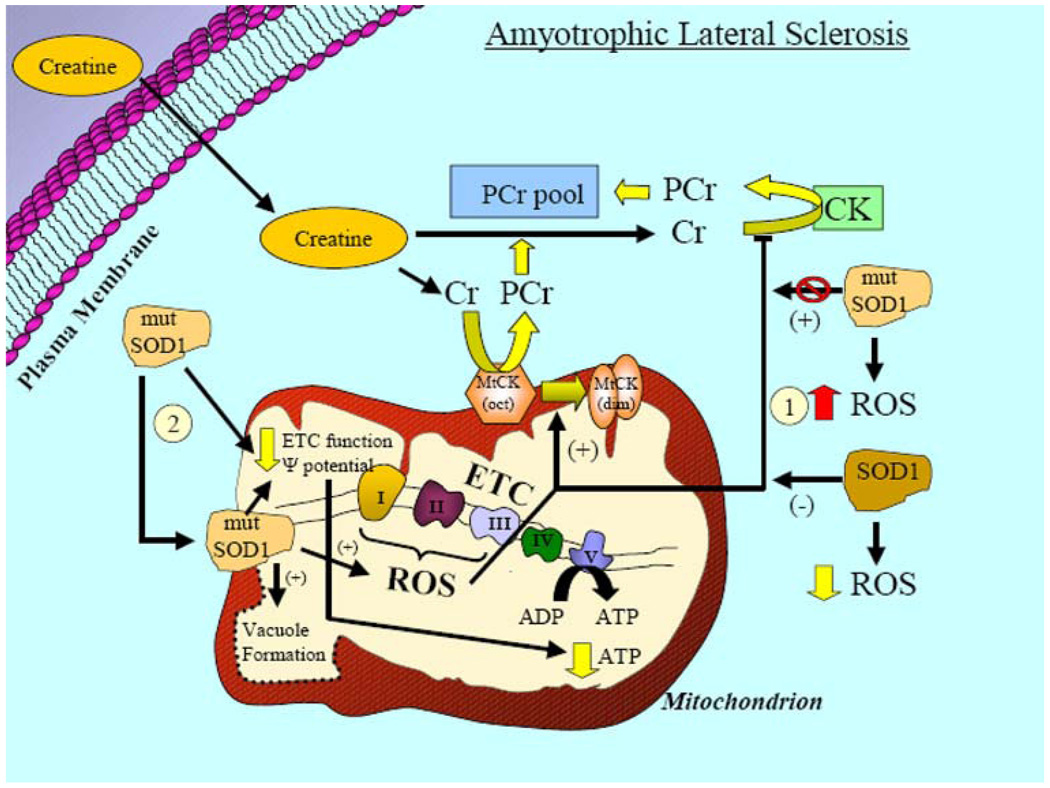
Fig. 4.
Schematic illustrating the relationship of mitochondria to amyotrophic lateral sclerosis (ALS) and the potential benefit of creatine supplementation. The most common form of familial ALS (FALS) is caused by numerous different mutations in the metallo-proteinase, superoxide dismutase I (SODI). SODI is an antioxidant that is ubiquitously expressed in the cytosolic fraction of cells and catalyzes the dismutation of superoxide (a highly reactive free radical) to the less reactive hydrogen peroxide (H2O2) and water. (1) Elevations in ROS may occur due to mutations in SOD1, causing reduced SOD1 antioxidant activity. Although somewhat counterintuitive, data have shown that this does not play a major role in the pathogenesis associated with SOD1 mutations. (2) Alternatively, the effects of mutant SOD1 are due to toxic gain-of-function properties. While SOD1 is primarily located in the cytosolic portion of the cell, mutant SOD1 has been found in the intermembrane space and the matrix of mitochondria. Although the detailed molecular mechanisms are not completely understood, mitochondrial mutant SOD1 is associated with impaired ETC function, reduced mitochondrial membrane potential, elevated mitochondrial ROS production, mitochondrial swelling, and intra-mitochondrial vacuole formation. These alterations likely contribute to reduced ATP generation by mitochondria and creatine supplementation in ALS may serve to buffer these mutant SOD1-induced impairments in intracellular bioenergetics. Creatine supplementation was shown to be neuroprotective in animal models of ALS (G93A), but despite promise, was ineffective in clinical trials with ALS patients