Abstract
Cadmium is a toxic heavy metal ranked seventh on the Priority List of Hazardous Substances. As a byproduct of smelters, cadmium is a prevalent environmental contaminant. It is also a major component of cigarette smoke, and its inhalation is associated with decreased pulmonary function, lung cancer, and chronic obstructive pulmonary disease. Ion channels, including the cystic fibrosis transmembrane conductance regulator (CFTR), play a central role in maintaining fluid homeostasis and lung functions. CFTR is mostly expressed in epithelial cells, and little is known about the effect of cadmium exposure on lung epithelial cell function. We show that exposure to cadmium decreases the expression of the CFTR protein and subsequent chloride transport in human airway epithelial cells in vitro. Impairment of CFTR protein expression was also observed in vivo in the lung of mice after intranasal instillation of cadmium. We established that the inhibitory effect of cadmium was not a nonspecific effect of heavy metals, as nickel had no effect on CFTR protein levels. Finally, we show that selected antioxidants, including alpha-tocopherol (vitamin E), but not N-acetylcysteine, can prevent the cadmium-induced suppression of CFTR. In summary, we have identified cadmium as a regulator of the CFTR chloride channel present in lung epithelial cells. Future strategies to prevent the deleterious effect of cadmium on epithelial cells and lung functions may benefit from the finding that alpha-tocopherol protects CFTR expression and function.
Keywords: CFTR, cadmium, airway epithelial cells, vitamin E
Cadmium, a highly toxic heavy metal, is a common air pollutant. It is released into the environment by the burning of coal, diesel fuel, gasoline and other fossil fuels, incineration of municipal waste, and from polluting metal alloy and electroplating facilities. Cadmium is currently ranked seventh on the Priority List of Hazardous Substances by the Agency for Toxic Substances and Disease Registry. This toxic heavy metal is also present in cigarette smoke and secondhand cigarette smoke. The lung more efficiently absorbs cadmium than the gastrointestinal tract (30–60% vs. 5–10%) (Jin et al., 2002; Nordberg, 2006). The lungs of smokers are exposed to very high concentrations of cadmium as each cigarette contains 1–3 μg of cadmium. As a consequence, an individual who smokes one pack of cigarettes a day will inhale around 40 μg of cadmium. The short-term (acute) effects of exposure to cadmium by inhalation are primarily seen in the lungs and are characterized by pneumonitis, bronchitis, and pulmonary edema. Owing to its long half-life (∼20–30 years), the long-term (chronic) inhalation or oral exposure to cadmium leads to its accumulation mainly in the kidneys where it can cause kidney disease (Jones and Cherian, 1990). An association between cadmium exposure and an increased risk of pulmonary diseases such as lung cancer and chronic obstructive pulmonary disease has been reported in humans and animals (Kirschvink et al., 2006; Nawrot et al., 2006; Nordberg, 2006). However, mechanisms underlying the adverse effects of cadmium on lung functions, including exchange of ions and gases, and motility of cilia remain poorly understood.
The Cystic Fibrosis Transmembrane conductance Regulator or CFTR is a chloride channel that resides at the apical membrane of airway epithelial cells where it plays a major role in maintaining the airway surface liquid and, therefore, regulates the physiology of the lung. In cystic fibrosis patients, CFTR dysfunction leads to impaired regulation of the airway surface volume and composition resulting in altered clearance of bacteria and chronic infection and inflammation (Boucher, 2004). In addition, it was recently reported that downregulation of CFTR, either via mutations in the cftr gene or via exposure to chemicals that inhibit its channel properties, induces a proinflammatory phenotype of the cells with increased sensitivity to releasing proinflammatory chemokines, such as IL-8 (Perez et al., 2007; Vij et al., 2009). Therefore, identifying conditions that could affect the expression and/or activity of the CFTR chloride channel is crucial to maintain proper lung function.
Although cadmium inhalation affects lung function, no study has addressed whether or not cadmium directly affects the CFTR ion channel expressed by airway epithelial cells. Because CFTR has to be present at the plasma membrane and functional to exert its role as a key regulator in fluid homeostasis in the airway, we wanted to investigate whether cadmium would alter CFTR expression and function. Our results indicate that cadmium, at doses that do not induce cell death, decreases the expression and subsequent activity of CFTR in various epithelial cell lines and primary cells as well as in vivo in mice. This effect is because of increase of CFTR ubiquitination/degradation. Interestingly, the antioxidant α-tocopherol (vitamin E) prevented the cadmium-induced inhibition of CFTR in bronchial epithelial cells and primary human airway epithelial cells (HAEC).
MATERIAL AND METHODS
Materials.
All chemicals including cadmium sulfate, cadmium chloride, α-tocopherol succinate (vitamin E), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma. Lactate dehydrogenase (LDH) leakage assay was performed using the TOX-7 cytotoxicity kit (Sigma). Cadmium sulfate was dissolved in water at a concentration of 100mM and was diluted in media (for in vitro studies) or PBS (for in vivo studies) to reach the indicated concentration.
Tissue culture and reagents.
The human bronchial epithelial cells Calu-3 were cultured in Dulbecco’s modified Eagle’s medium/F12 supplemented with 10% (vol/vol) bovine growth serum and penicillin/streptomycin and grown at 37°C (5% CO2). Experiments were performed using Calu-3 cells between passages 25 and 50. Transformed human bronchial epithelial cells 16HBE, kindly donated by Dr Gruenert, were grown in minimum essential media (InVitrogen) containing 10% fetal bovine serum, 2mM L-glutamine (InVitrogen), and penicillin/streptomycin (InVitrogen) on collagen-coated (BD Biosciences) and fibronectin-coated (BD Biosciences) dishes. Primary HAEC were isolated after enzymatic dissociation from trachea, bronchi, and bronchioles of adult donor lungs, seeded onto collagen-coated semipermeable membranes (0.6 cm2, Millicell-HA; Millipore, Bedford, MA), and grown at an air-liquid interface as previously described (Bao et al., 2005; Karp et al., 2002). Human lungs were collected with approval from The Ohio State University Institutional Review Board.
Immunoprecipitation and immunoblotting.
Cells were lysed in PBS containing 1% Triton X-100 and a cocktail of protease inhibitors (Roche). The lysates were subjected to immunoprecipitation (IP) using C-CFTR monoclonal antibody (mAb 24-1; R and D Systems) cross-linked to protein A/G agarose beads. After extensive washes with PBS/1% Triton X-100, the bound proteins were analyzed by immunoblotting following transfer to polyvinylidenedifluoride membranes (BioRad) as previously described (Cormet-Boyaka et al., 2004; Rennolds, Boyaka, et al., 2008). Membranes were blocked with PBS 5% dry milk and primary antibodies were added at 1:2000 (C-CFTR; R and D Systems), 1:5000 (β-actin; Santa Cruz Biotechnology), 1:1000 (metallothionein; Abcam), 1:1000 (SLC11A2; GeneTex), or 1:2000 (ubiquitin; Santa Cruz Biotechnology) dilutions, respectively. Secondary antibodies (horseradish peroxidase conjugated; Pierce, Rockford, IL) were diluted 1:10,000. Chemoluminescent signal was detected using West Pico (Pierce) following the manufacturer’s instructions. The intensity of the bands was estimated by Quantity One software (BioRad).
Animals and lung tissue processing.
C57BL/6 mice (8–10 weeks old) were used in accordance with the institutional animal welfare guidelines of the Ohio State University of Columbus. Anesthetized mice received 10 nmol cadmium (in 50 μl PBS) or vehicle (control mice) by intranasal instillation. Three days later, mice were sacrificed and lung tissues were snap frozen in liquid nitrogen for protein processing and stored at 80°C until assayed. Frozen specimens (−80°C) were transferred into liquid nitrogen and shattered in a BioPulverizer (Biospec Products, Bartlesville, OK). Tissue fragments were suspended in PBS containing 1% Triton X-100 and a cocktail of protease inhibitors (Roche) to extract the proteins. The protein concentration was determined by BCA protein assay (Pierce).
Cell surface biotinylation.
The Calu-3 cells were rinsed with ice-cold PBS containing 0.1mM CaCl2 and 1mM MgCl2 to eliminate the proteins present in the media. Because the biotinylation reagent cross-links primarily through lysine residues, residual serum will inhibit cell surface biotinylation. Cell surface proteins are labeled with 1 mg/ml EZ-Link NHS-SS Biotin (Pierce) for 30 min at 4°C. Following biotinylation, cells were rinsed with PBS containing 1% bovine serum albumin for 10 min at 4°C to quench any residual NHS-SS biotin. At the end of the experiments, the cells were lysed with PBS-1% Triton X-100 and protease inhibitors (Roche). Biotinylated proteins were incubated with streptavidin beads overnight at 4°C. After extensive washings, bound proteins were subjected to Western blot analysis. Biotinylated CFTR was detected using a C-CFTR monoclonal antibody (mAb, 24-1; R and D Systems).
Measurement of cell viability.
Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Cells were treated with 10–200μM cadmium for 4 days. At the end of the treatment, the number of viable cells was determined by measuring their capacity to convert a tetrazolium salt into a blue formazan product.
Transepithelial short-circuit currents.
Calu-3 cells and primary HAEC were cultured on permeable membranes (Transwell from Costar for Calu-3 cells and Millicell-HA from Millipore for primary HAEC). Cells were treated with cadmium or other effectors for 5 days and filters were mounted in Ussing chambers. The cyclicAMP (cAMP)-induced short-circuit currents (Isc) were recorded as previously described (Rennolds, Tower, et al., 2008). The experiments with the primary HAEC were performed in the presence of amiloride to inhibit the epithelial sodium channel ENaC. The Calu-3 cells, which do not express ENaC, were not treated with amiloride.
Quantitative real-time PCR analysis.
Quantitative real-time PCR (qRT-PCR) was employed to measure the transcript levels of the cftr gene. First-strand complementary DNAs (cDNAs) were synthesized using SS Reverse Transcriptase (InVitrogen) from 2 to 4 μg of total RNA in a 20 μl reaction volume. Quantitative PCR was performed with SYBR green I PCR master mixture in the PRISM 7700 apparatus (Applied Biosystems). PCR amplification reactions were initially incubated at 94°C for 5 min followed by 40 cycles at 94°C for 30 s, 62°C for 1 min, and 72°C for 1 min. RT-PCR for amplifying transcripts of the cftr gene was performed at least three times to confirm the accuracy of the results. The total synthesized cDNA were also used to amplify the cyclophilin and CAP-1 genes as internal standards using cyclophilin and CAP-1 gene–specific primers. The CFTR and ZIP8 messenger RNA (mRNA) levels were normalized to the expression of the housekeeping genes cyclophilin and CAP-1 (cAMP-accessory protein) and expressed as relative expression (RE), which were calculated using the following equation: RE = 2−ΔΔCt, where ΔCt = Ct(target) − Ct(endogenous controls) and ΔΔCt = ΔCt(treated) − ΔCt(control).
Statistical analysis.
Data are expressed as mean ± SEM of at least three independent experiments. Statistically significant differences were assessed using Student’s t-test. p values < 0.05 were considered significant.
RESULTS
Cadmium Decreases the Expression of the CFTR Protein
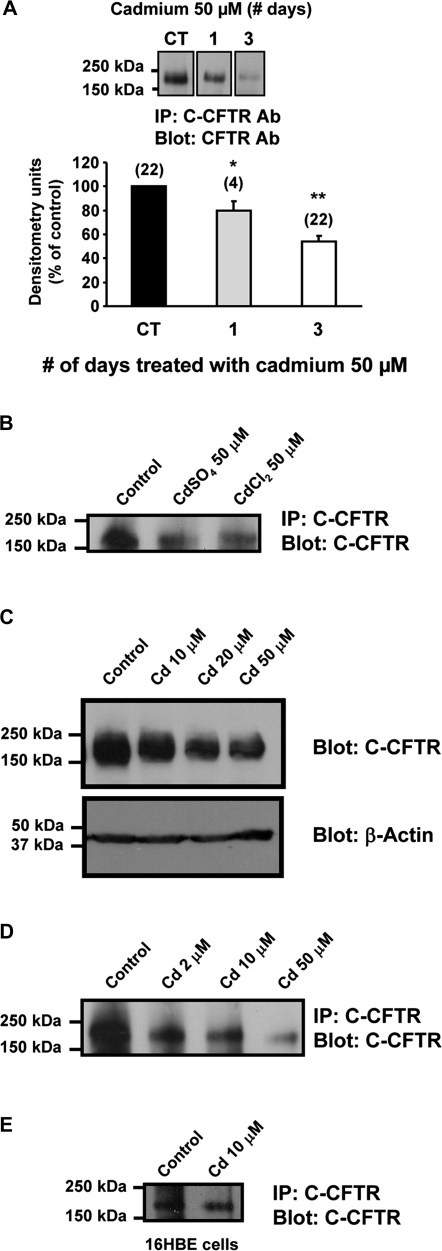
We initially investigated the effect of cadmium on the expression of CFTR in HAEC. As cadmium concentration was estimated to be around 30–50μM in the lung of smokers (Paakko et al., 1988), 50μM cadmium sulfate (cadmium) was added to confluent Calu-3 cells. Cadmium was added for 1 and 3 days before being harvested. Control cells have been incubated without cadmium for 3 days. As shown in Figure 1A, treatment with cadmium progressively reduced the amounts of CFTR protein down to 54 ± 5% of control in Calu-3 cell extracts. Similar results were obtained with cadmium chloride indicating that the responses were not because of the sulfate component of cadmium sulfate (Fig. 1B). We also found that at the concentration of 50μM, cadmium had no effect on the membrane protein divalent metal ion transporter (DMT1), which carries iron but can also transport cadmium (Bannon et al., 2003) (data not shown). This latter result suggests that cadmium does not affect all membrane proteins. A decrease in CFTR protein was also observed when Calu-3 cells were incubated for 3 days with 10–50μM cadmium (Fig. 1C). We found that exposure to lower doses of cadmium (2μM) for 3 days did not affect levels of CFTR protein (data not shown). On the other hand, Figure 1D clearly shows that there is a dose-dependent decrease of CFTR protein with increasing doses of cadmium. It also shows that the 2μM dose of cadmium could reduce the expression of CFTR protein by Calu-3 cells but after 5 days of exposure. This lag in the effect of the lower cadmium dose is probably because of a need for sufficient uptake and/or accumulation of this heavy metal by the cells. In summary, the inhibitory effect of cadmium on CFTR expression in Calu-3 cells appears to be dose and time dependent (Figs. 1C and 1D). To address whether the effect of cadmium was restricted to Calu-3 cells, HAEC 16HBE were exposed to 10μM cadmium for 3 days. As seen with Calu-3 cells, cadmium also inhibited CFTR expression in these cells (Fig. 1E).
FIG. 1.
Cadmium decreases the expression of the CFTR protein. (A) Time course of CFTR expression following exposure to cadmium in the human bronchial epithelial cell line Calu-3. Cadmium sulfate (50μM) was added 1 or 3 days before harvesting the cells. Control cells were incubated with vehicle for 3 days. CFTR was detected as described in “Materials and Methods” section. Data are expressed as mean ± SEM. Number of experiments (n). *p < 0.05; **p < 0.001. (B) Both cadmium sulfate (CdSO4) and cadmium chloride (CdCl2) decrease the expression of the CFTR protein. Calu-3 cells were incubated with 50μM CdSO4 or CdCl2 for 3 days. (C and D) Cadmium decreases the expression of the CFTR protein in a dose-dependent manner. Calu-3 cells were incubated for 3 days with 10–50μM cadmium (C) or 5 days with 2–50μM cadmium (D). (E) Cadmium decreases the expression of the CFTR protein in the human bronchial epithelial cells 16HBE. 16HBE cells were exposed to 10μM cadmium for 3 days. CFTR protein was detected using anti-C-CFTR antibody as described in the “Materials and Methods” section. Data are representative of at least three independent experiments.
Cadmium Decreases CFTR-Mediated Chloride Transport
We next investigated whether or not the inhibitory effect of cadmium on CFTR expression also affected the function of this channel. Control and cadmium-treated polarized monolayers of bronchial epithelial cells (Calu-3) were mounted in Ussing chambers and cAMP-induced short-circuit currents (Isc) were measured upon addition of forskolin (that increases intracellular cAMP). At the end of the experiment, a CFTR inhibitor was added to confirm that the currents were CFTR mediated. Calu-3 cells treated with 50μM cadmium displayed 38.9 ± 6.3% decrease in CFTR-mediated currents indicating that cadmium affects CFTR activity on polarized cells.
Effect of Cadmium Cytotoxicity
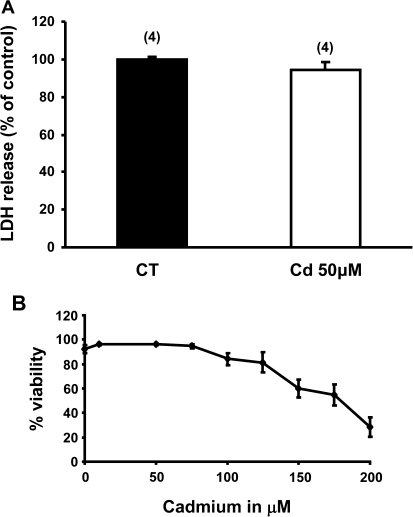
It was important to establish that the inhibitory effect of cadmium on CFTR expression and activity was not a consequence of the cytotoxicity of this heavy metal for epithelial cells. Analysis of culture medium of control Calu-3 cells or Calu-3 cells treated with 50μM cadmium showed no significant release of LDH indicating that this dose of cadmium does not appear to induce necrotic cell death (Fig. 2A). This finding was further confirmed by MTT assay in which Calu-3 cells were exposed to cadmium doses up to 200μM (Fig. 2B). Indeed, Calu-3 cells treated with up to 50μM cadmium had viability similar to untreated control cells (Fig. 2B). However, a significant decrease in cell viability was observed in Calu-3 cultures exposed to concentrations of cadmium of at least 150μM (Fig. 2B).
FIG. 2.
Cadmium 50μM does not appear to induce necrotic cell death in Calu-3 cells. (A) Calu-3 cells were exposed to 50μM cadmium for 3 days. Cadmium cytotoxicity was evaluated by measuring LDH release in the culture medium as described in “Materials and Methods” section. Data are expressed as mean ± SEM of four independent experiments. (B) Calu-3 cells were exposed to 10–200μM cadmium for 4 days. Cell viability was evaluated using the MTT assay as described in “Materials and Methods” section. Data are expressed as mean ± SEM of at least three independent experiments.
Cadmium Decreases the Expression of the CFTR Protein in the Lung of Mice
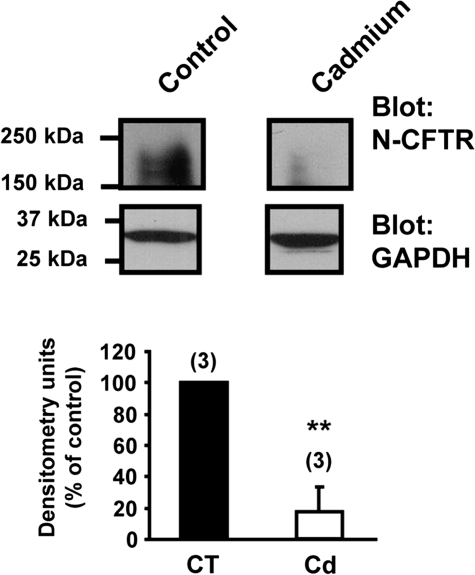
Finally, we investigated if the inhibitory effect of cadmium on CFTR is an event that could occur in mice in vivo. For this purpose, groups of mice received cadmium (10 nmol in 50 μl PBS) or PBS (control) via intranasal instillation and lungs harvested 3 days later. This dose of cadmium was previously reported to not induce injury or cell death in mice (Pearson et al., 2003). Furthermore, analysis of lung tissues showed over 80% decrease in CFTR protein expression in mice treated with cadmium (Fig. 3).
FIG. 3.
Cadmium decreases the expression of the CFTR protein in the lung of mice. Three mice received either 10 nmol of cadmium in PBS or PBS (control) by intranasal instillation. Three days later, the lungs were harvested and processed as described in “Materials and Methods” section. CFTR protein present in 100 μg total proteins was detected by immunoblotting using N-CFTR polyclonal antibody (Cell Signaling Technology). The housekeeping gene GAPDH showed that an equal amount of protein was loaded in each lane. **p < 0.001.
Cadmium Decreases CFTR Levels on the Plasma Membrane of Epithelial Cells
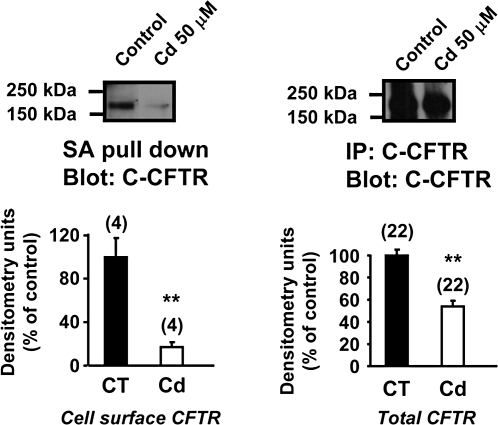
In order to verify that the cadmium-induced decrease in total CFTR protein levels affected CFTR levels at the plasma membrane (site where CFTR exerts its chloride channel function), cell surface proteins were biotinylated and pulled down using streptavidin beads. Western blot analysis with CFTR-specific antibody showed a strong decrease (≈90%) in the CFTR signal representing membrane-associated CFTR protein in cadmium-treated cells as compared with untreated (control) cells (Fig. 4), in conditions where the same amount of total protein was incubated with streptavidin beads. In order to confirm that biotin does not penetrate inside the cells that would result in labeling of intracellular proteins as well, we evaluated the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the streptavidin pull down. Only a small amount of GAPDH was detected in the streptavidin pull down that corresponds to nonspecific binding of GAPDH to streptavidin beads (data not shown). These results indicate that cadmium mainly affects the levels of plasma membrane CFTR.
FIG. 4.
Cadmium decreases plasma membrane CFTR. Calu-3 cells were exposed to 50μM cadmium for 3 days. Plasma membrane proteins were labeled with biotin and the same amount of total protein was incubated with streptavidin beads as described in the “Materials and Methods” section. CFTR present in cell lysates was detected using anti-C-CFTR monoclonal antibody (24-1, R&D Systems). Data are expressed as mean ± SEM. Number of experiments (n). **p < 0.001.
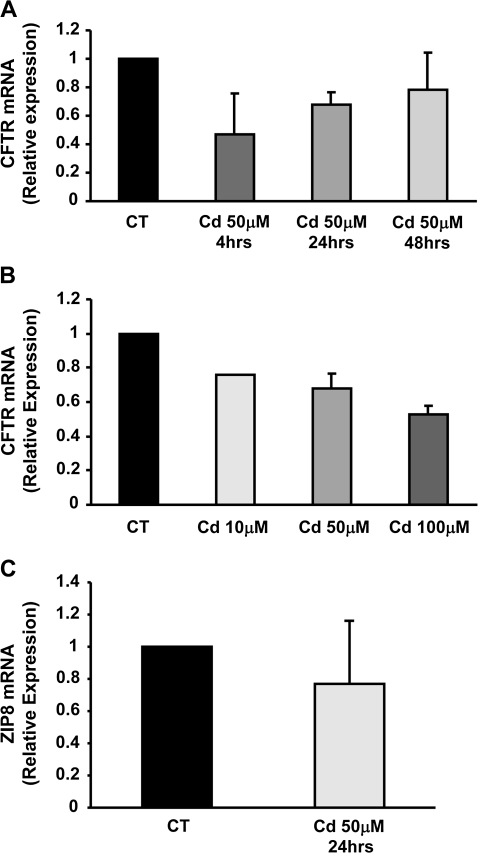
Effect of Cadmium on CFTR mRNA Transcript Levels
In order to better understand the mechanism of cadmium-induced inhibition of the CFTR protein, we quantified mRNA transcript levels of CFTR in cells treated with cadmium using qRT-PCR. The levels of expression of CFTR and ZIP8 mRNA were normalized to the expression of two housekeeping genes (cyclophilin and CAP-1). Treatment with 50μM cadmium for 4–48 h resulted in a transient reduction of CFTR mRNA levels in Calu-3 cells (Fig. 5A). We then investigated the effect of various concentrations of cadmium (10–100μM) on CFTR mRNA levels. We observed that 24-h treatment with cadmium resulted in a concentration-dependent decrease of CFTR mRNA levels. Finally, the effect of cadmium on the mRNA levels of the zinc transporter ZIP8 was examined. ZIP8 is expressed at the apical surface of epithelial cells, like CFTR, and has been reported to transport cadmium (Liu et al., 2008). Incubation of Calu-3 cells with 50μM cadmium for 24 h had no effect on ZIP8 mRNA transcript levels (Fig. 5C). In summary, cadmium also affected CFTR mRNA levels in epithelial cells, although to a lesser extent than its effect on CFTR protein levels.
FIG. 5.
Effect of cadmium on CFTR mRNA transcript levels in Calu-3 cells. (A) Calu-3 cells were incubated with 50μM cadmium for 4–48 h. (B) Calu-3 cells were incubated for 24 h with 10–100μM cadmium. (C) qRT-PCR analysis of ZIP8 mRNA transcript levels were analyzed after treatment of Calu-3 cells with 50μM cadmium for 24 h. CFTR and ZIP8 mRNA transcript levels were measured as described in “Materials and Methods” section. Data are expressed as mean ± SEM of at least three independent experiments.
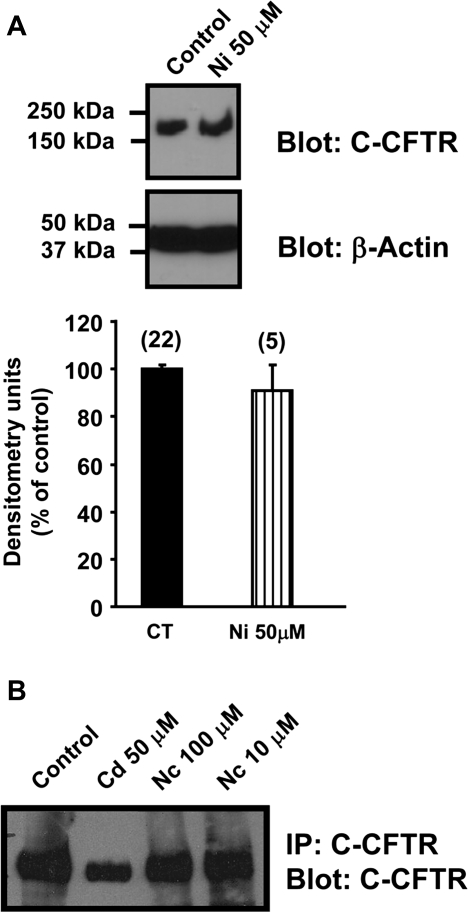
The Heavy Metal Nickel and Nornicotine (a metabolite of nicotine) Have No Effect on CFTR Protein
We investigated whether the observed effects of cadmium on CFTR were specific to this heavy metal or whether other heavy metals were capable of altering CFTR expression in epithelial cells. For these studies, we chose nickel because like cadmium, nickel is an environmental and occupational health hazard, and its inhalation is associated with lung pathology, such as acute lung injury and lung cancer (Leikauf et al., 2002; Wild et al., 2009). Calu-3 cells were exposed to 50μM nickel for 3 days. Interestingly, Calu-3 cells exposed to 50μM nickel for 3 days showed no alteration of CFTR protein expression (Fig. 6A), suggesting that not all heavy metals affect CFTR. As cadmium is abundantly present in cigarette smoke, we tested the effect of nornicotine, a metabolite of the cigarette smoke component nicotine, on the CFTR protein. Addition of 10 or 100μM nornicotine for 3 days had no effect on the CFTR protein as observed in Figure 6B.
FIG. 6.
The heavy metal nickel and nornicotine (a metabolite of nicotine) have no effect on CFTR protein. (A) The human bronchial epithelial cells Calu-3 were incubated for 3 days with nickel (50μM). Data are expressed as mean ± SEM. Number of experiments (n). (B) Calu-3 cells were incubated with 50μM cadmium or 10 and 100μM nornicotine for 3 days. CFTR protein was detected as described in the “Materials and Methods” section. Data are representative of at least three independent experiments.
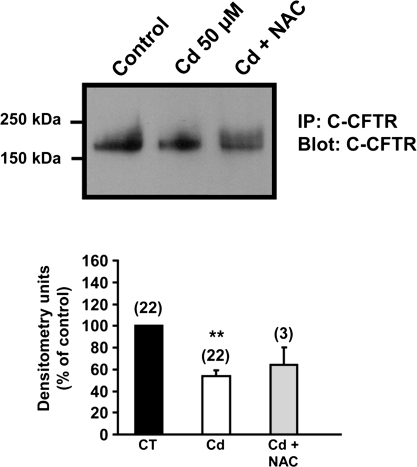
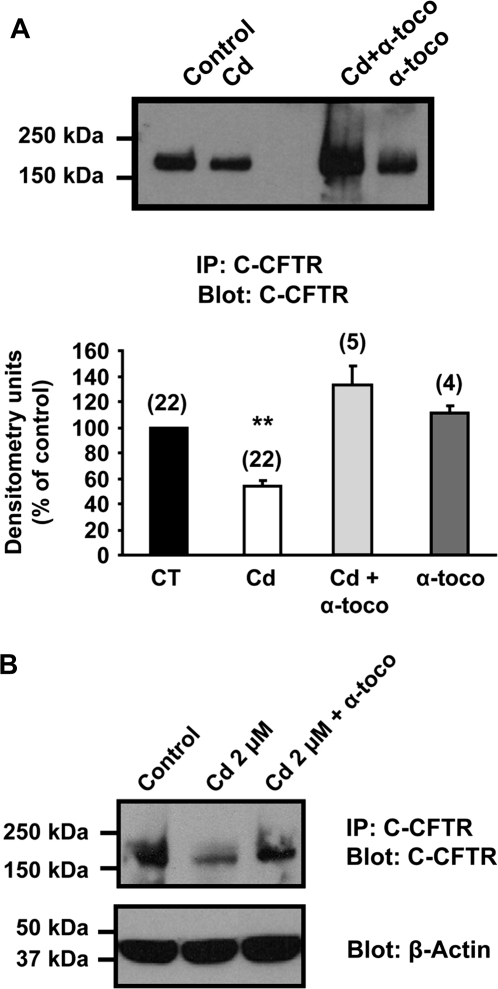
The Antioxidant α-Tocopherol (Vitamin E) Prevents the Cadmium-Induced Inhibition of the CFTR Protein
Our results so far indicate that cadmium decreases CFTR at the protein level and to some extent at the mRNA level. Because cadmium primarily affected cell surface levels of CFTR, we next tested the hypothesis that cadmium exerted its effect on CFTR via induction of oxidative stress and thus protein degradation. Cadmium is not a Fenton metal and therefore is not able to generate reactive oxygen species (ROS), but it can induce production of free radicals (Han et al., 2007; Liu et al., 2009). We therefore tested the effects of the antioxidant N-acetylcysteine (NAC), which can act as a direct scavenger of ROS. These experiments were performed in Calu-3 cells treated for 3 days with 50μM cadmium (see Fig. 1). NAC did not prevent the decrease of CFTR protein obtained after cadmium treatment (Fig. 7). We next tested whether the liposoluble antioxidant α-tocopherol (vitamin E) could protect the CFTR protein from degradation by the cadmium treatment. Unlike NAC, α-tocopherol could prevent the cadmium-induced inhibition of CFTR expression in the human bronchial epithelial cells Calu-3 (Fig. 8A). In control experiments, α-tocopherol was added alone to the cells and no change in CFTR protein could be observed. The protective effect of α-tocopherol was also observed when Calu-3 cells were incubated for 5 days with 2μM cadmium (Fig. 8B).
FIG. 7.
The antioxidant NAC does not prevent cadmium-induced reduction of CFTR protein in Calu-3 cells. Calu-3 cells were incubated for 3 days with 50μM cadmium (Cd) alone or in combination with NAC (10mM). CFTR was detected using anti-C-CFTR antibody (24-1; R and D Systems) as described in the “Materials and Methods” section. **p < 0.001.
FIG. 8.
The antioxidant α-tocopherol (vitamin E) prevents cadmium-induced reduction of CFTR protein in Calu-3 cells. Calu-3 cells were incubated for 3 days with 50μM cadmium (Cd) (A) or for 5 days with 2μM cadmium (B) alone or in combination with α-tocopherol (200μM). CFTR was detected using anti-C-CFTR antibody (24-1; R and D Systems) as described in the “Materials and Methods” section. **p < 0.001.
The Antioxidant α-Tocopherol (Vitamin E) Prevents the Cadmium-Induced Inhibition of CFTR-Mediated Chloride Transport
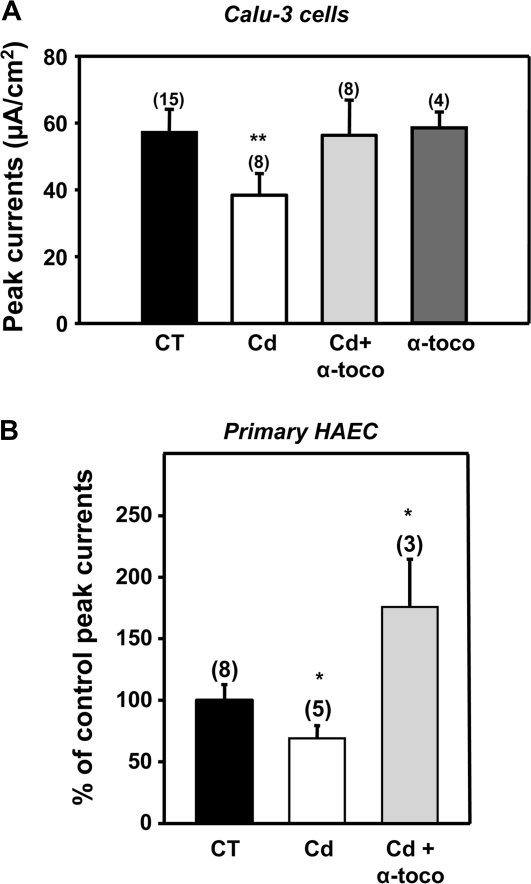
Next, we studied the effect of α-tocopherol on the cadmium-induced inhibition of CFTR-mediated chloride transport. As shown in Figure 9A, Calu-3 cells treated for 5 days with 50μM cadmium displayed 38.9 ± 6.3% decrease in CFTR-mediated currents indicating that cadmium affects CFTR function in polarized cells. Addition of α-tocopherol together with cadmium prevented the inhibitory effect of cadmium on CFTR function, whereas α-tocopherol alone had no effect on CFTR function (Fig. 9A). In another set of experiments, cadmium (50μM) was added directly to the chamber containing activated-CFTR but no inhibition of chloride transport was observed (data not shown). This latter result indicates that cadmium does not have an acute effect and does not act as a CFTR channel blocker at this dose. The transepithelial resistance was similar in control and treated cells indicating that cadmium did not affect the tight junctions.
FIG. 9.
The antioxidant α-tocopherol (vitamin E) prevents the cadmium-induced inhibition of CFTR activity in bronchial epithelial cells Calu-3 and primary HAEC. (A) Histogram representation of peak currents representing cAMP-activated short-circuit currents (Isc) in Calu-3 cells. Calu-3 monolayers were incubated with 50μM cadmium (Cd) and/or 200μM α-tocopherol for 5 days. Calu-3 cells were grown on filters and polarized monolayers were mounted in Ussing chambers to measure ion transport. cAMP-activated chloride currents, that reflect CFTR activity, were recorded following addition of forskolin (20μM) that increases intracellular cAMP. (B) Primary HAEC were incubated with 50μM cadmium (Cd) and/or 200μM α-tocopherol for 5 days. Histogram representation of percentage control peak currents representing cAMP-activated short-circuit currents (Isc). Data are expressed as mean ± SEM. Number of experiments (n). *p < 0.01; **p < 0.001.
We also used primary HAEC to record inhibitory effects of cadmium on CFTR-mediated chloride currents. Primary HAEC were treated for 5 days with 20μM cadmium instead of 50μM because this latter concentration disturbed the integrity of the monolayer as assessed by transepithelial resistance measurements. We found that CFTR-mediated currents were decreased by ∼30% in primary HAEC treated with 20μM cadmium for 5 days (Fig. 9B). As observed with Calu-3 cells, addition of α-tocopherol prevented the cadmium-induced inhibition of CFTR-mediated chloride transport and even resulted in a slight increase over control condition.
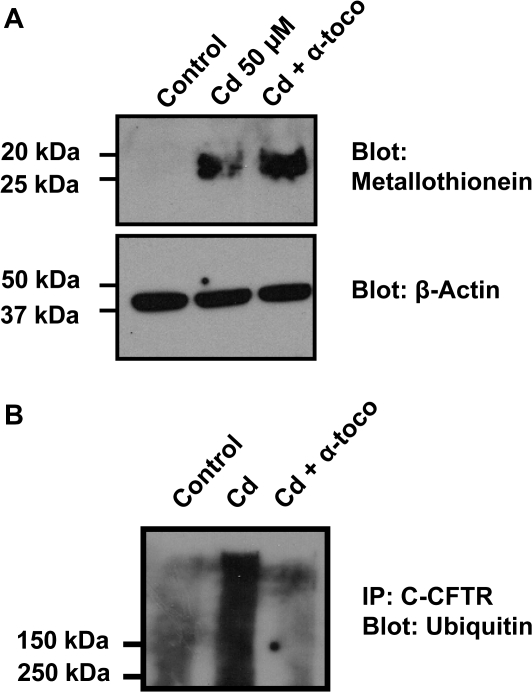
Effect of α-Tocopherol on the Cadmium-Scavenger Protein, Metallothionein
In order to understand the mechanism by which α-tocopherol prevents the effect of cadmium on the CFTR chloride channel, the expression of the metal-induced protein metallothionein was monitored. As cadmium enters the cell, the synthesis of metallothionein is increased as it serves as a scavenger for intracellular cadmium ions (Nzengue et al., 2009). Accordingly, we failed to detect metallothionein in cells incubated in a medium without cadmium (Fig. 10A). Addition of cadmium to the medium triggered a strong increase in metallothionein protein signal that could be detected with a similar intensity in presence of α-tocopherol. These results suggest that α-tocopherol does not alter induction of metallothionein triggered by cadmium.
FIG. 10.
Effect of the antioxidant α-tocopherol on metallothionein synthesis and ubiquitination of CFTR. (A) Cadmium induces synthesis of metallothionein. Metallothionein proteins were detected in cell lysates from cells treated for 3 days with cadmium (50μM) ± α-tocopherol (200μM). The membranes were reprobed with β-actin antibody to show that the same amount of protein was loaded in each lane. CFTR protein was detected as described in the “Materials and Methods” section. Data are representative of at least three independent experiments. (B) Cadmium induces ubiquitination of CFTR. Calu-3 cells were treated with 50μM cadmium ± 200μM α-tocopherol. CFTR present in cell lysate was immunoprecipitated using a C-CFTR antibody and the blot was subjected to immunoblotting using a monoclonal antibody that recognizes ubiquitin. Data are representative of at least three independent experiments.
α-Tocopherol Prevents the Cadmium-Induced Ubiquitination of CFTR
Cadmium treatment was previously reported to result in accumulation of high–molecular weight polyubiquitinated proteins. Because the CFTR protein is degraded through the ubiquitin (ub) proteasome pathway, we wanted to know whether cadmium increases ubiquitination of CFTR. Calu-3 cells were treated with cadmium for 24 h, and cell lysates were subjected to IP using a C-CFTR antibody. As observed in Figure 10B, cadmium treatment resulted in accumulation of ub-CFTR. Preteatment of the cells with the antioxidant α-tocopherol precluded accumulation of such complex (Fig. 10B). These results indicate that cadmium induces ubiquitination of the CFTR protein that was prevented by the antioxidant α-tocopherol.
DISCUSSION
It is now well established that cadmium inhalation leads to several lung pathologies. Epithelial cells play a central role in lung physiology and represent the first line of cells encountered by exogenous molecules in the lung. Surprisingly, little is known about the effect of cadmium on ion channels present in airway epithelial cells. Data presented herein show that subcytotoxic concentration of cadmium exerts a direct effect on a key function of airway epithelial cells. Thus, cadmium inhibits the levels of expression of the CFTR chloride channel resulting in a decrease in chloride transport in HAEC. Another important finding from our studies is the identification of α-tocopherol as a lipophilic antioxidant capable of preventing alteration of CFTR function induced by cadmium.
Environmental pollutants are known to induce several lung pathologies, but the mechanisms underlying alteration of lung functions by cadmium are only partially understood. Alteration of epithelial cell functions caused by mutations in the cftr gene can cause cystic fibrosis, a disease characterized by chronic infection and inflammation of the airways. CFTR is an ion channel that plays a key role as regulator of fluid homeostasis in the airway. This is the first report to link exposure to cadmium with alteration of the expression of the CFTR chloride channel in epithelial cells. Previous studies by others have shown that cadmium can regulate the activity of some ion channels present in epithelial cells by interfering with the pore of the channels. For example, cadmium has been reported to inhibit the epithelial sodium channel ENaC as well as the pH-activated ClC-2 chloride channel because of direct binding of cadmium to the channels at high concentrations (Blaisdell et al., 2000; Chu et al., 2004; Takeda et al., 2007). Cadmium has also been reported to inhibit CFTR activity albeit at very high doses (1–5mM) (Annereau et al., 2003; Harrington et al., 1999). A report by Harrington et al. (1999) suggested that cadmium modifies the opening and closing rates of the CFTR chloride channel in HEK293 cells stably transfected with human CFTR. Conversely, lower doses of cadmium were recently reported to increase CFTR activity (L’Hoste et al., 2009). In our experiments, we observed that 50μM cadmium decreases CFTR-mediated chloride transport. This difference might be because of the length of cadmium treatment, days in our study versus minutes in L’Hoste et al.’s (2009) study. Interestingly, this latter study shows that the presence or absence of functional CFTR influences whether the response of proximal tubule cells to cadmium exposure will consist of apoptosis or necrosis. Furthermore, previous reports revealed that downregulation of CFTR in HAEC increased their ability to release proinflammatory chemokines, such as IL-8 (Perez et al., 2007; Vij et al., 2009). Our data clearly indicate that cadmium affects CFTR protein expression as we observed ∼50% decrease in total CFTR protein and ∼80% decrease in plasma membrane CFTR in cadmium-treated cells. Taken together, these results suggest that cadmium could modify the physiology of epithelial cells. This latter point is currently under investigation in our laboratory.
Factors that influence the activity of CFTR include the synthesis of CFTR protein, its intracellular trafficking, and stability at the cell surface. In our study, plasma membrane CFTR was reduced by 80% upon cadmium treatment of epithelial cells. Unexpectedly, only a 40% decrease in CFTR activity could be observed after CFTR activation with forskolin. It has been reported that stimulation of Madin-Darby canine kidney cells with forskolin leads to insertion of CFTR molecules into the apical plasma membrane (Howard et al., 2000). Our experiments to assess plasma membrane CFTR were performed in unstimulated cells, whereas CFTR activity was determined in forskolin-stimulated cells. It is therefore possible that a pool of CFTR molecules present near the plasma membrane could be delivered to the apical membrane of polarized Calu-3 when assessing CFTR activity, and this could account for the lower inhibition observed. We also observed that delivery of cadmium to the lung of mice resulted in over 80% suppression of the CFTR protein. It is important to note that the dose of cadmium (10 nmol) delivered to the mice in vivo corresponds to 1/10 of the dose used on epithelial cells in the in vitro studies (with 50μM cadmium corresponding to 100 nmol cadmium). These results indicate that cadmium-induced suppression of the CFTR protein in vivo in mice is probably because of both direct and indirect mechanisms.
The major source of cadmium in the lung of smokers is from cigarette smoke and is estimated to be around 50–30μM (Chambers et al., 1998; Paakko et al., 1988, 1989). Welsh’s (1983) laboratory was the first to report the inhibitory effect of cigarette smoke on chloride transport using a tracheal canine model. Furthermore, it was recently reported that exposing human bronchial epithelial cells to cigarette smoke resulted in a decrease in chloride transport that was associated with inhibition of the expression of CFTR chloride channel (Cantin et al., 2006; Kreindler et al., 2005). Cantin et al. (2006) showed that cigarette smoke decreased the expression and function of CFTR in vitro and that cigarette smokers exhibited nasal potential differences resembling those of patients with cystic fibrosis (lacking functional CFTR chloride channels). We have tested the effect of nornicotine, a metabolite of the cigarette smoke component nicotine, and found no effect on CFTR protein (see Fig. 6B). Because cigarette smoke is an important source of cadmium, our data suggest that cadmium could be a key component of cigarette smoke responsible for the suppression of CFTR function. Such concept is currently being investigated in our laboratory.
Cadmium inhalation and ingestion have been associated with kidney disease. On the other hand, it is well established that the CFTR protein is expressed in the kidney where it regulates fluid secretion by interacting with other ion channels, such as renal outer medullary potassium channel and ENaC (Konstas et al., 2002; Lu et al., 2006). However, the physiological role of CFTR in the kidney is not clear. CF patients did not traditionally develop major kidney diseases, but as their life expectancy increases, there are more and more reports of nephropathy (Abramowsky and Swinehart, 1982; Melzi et al., 1991). Though nephropathy could be because of long-term exposure of renally toxic antibiotics or immunosuppressants, it could also result from nonfunctional CFTR (Soulsby et al., 2009). It would therefore be important to examine the effect of cadmium on the expression/function of CFTR in kidney cells.
This study also allowed us to make the important observation that α-tocopherol, a form of vitamin E, can prevent the inhibition of CFTR protein as well as activity (Figs. 8 and 9). This observation is crucial because α-tocopherol, but not NAC, could counter the deleterious effect of cadmium on CFTR. α-Tocopherol, a fat-soluble antioxidant, prevents lipid peroxidation and therefore participates in preservation of membrane integrity. Its two major roles in membranes are as a lipid-soluble antioxidant that acts to prevent free radical damage of polyunsaturated fatty acids and as a membrane-stabilizing agent through its van der Waals interaction with membrane phospholipids. It is therefore possible that oxidation of the fatty acids in the region where the CFTR protein resides could alter its stability. In this regard, we observed an increase in ubiquitination of CFTR complex because of addition of cadmium that was prevented by α-tocopherol pretreatment (Fig. 10). Our finding is also consistent with the fact that vitamin E has previously been reported to protect animals and cells against cadmium-induced toxicity (Koyuturk et al., 2007; Warren et al., 2000).
In conclusion, our data show that cadmium decreases the abundance of CFTR at the plasma membrane resulting in a decrease in chloride transport in epithelial cells present in the lung. The liposoluble antioxidant α-tocopherol prevents the loss of surface CFTR induced by this agent. Because CFTR plays a key role in maintaining lung homeostasis, it would be important to investigate its role in the development of pulmonary diseases other than cystic fibrosis.
FUNDING
National Institutes of Health (RO3HL095442 to E.C.-B.).
Acknowledgments
The authors would like to thank Dr Mikhail Gavrilin for helping with quantification of mRNA transcript levels.
References
- Abramowsky CR, Swinehart GL. The nephropathy of cystic fibrosis: a human model of chronic nephrotoxicity. Hum. Pathol. 1982;13:934–939. doi: 10.1016/s0046-8177(82)80056-7. [DOI] [PubMed] [Google Scholar]
- Annereau JP, Ko YH, Pedersen PL. Cystic fibrosis transmembrane conductance regulator: the NBF1+R (nucleotide-binding fold 1 and regulatory domain) segment acting alone catalyses a Co2+/Mn2+/Mg2+-ATPase activity markedly inhibited by both Cd2+ and the transition-state analogue orthovanadate. Biochem. J. 2003;371:451–462. doi: 10.1042/BJ20021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon DI, Abounader R, Lees PS, Bressler JP. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am. J. Physiol. Cell. Physiol. 2003;284:C44–C50. doi: 10.1152/ajpcell.00184.2002. [DOI] [PubMed] [Google Scholar]
- Bao S, Wang Y, Sweeney P, Chaudhuri A, Doseff AI, Marsh CB, Knoell DL. Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L36–L42. doi: 10.1152/ajplung.00309.2003. [DOI] [PubMed] [Google Scholar]
- Blaisdell CJ, Edmonds RD, Wang XT, Guggino S, Zeitlin PL. pH-regulated chloride secretion in fetal lung epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L1248–L1255. doi: 10.1152/ajplung.2000.278.6.L1248. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am. J. Respir. Crit. Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- Chambers RC, Laurent GJ, Westergren-Thorsson G. Cadmium inhibits proteoglycan and procollagen production by cultured human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 1998;19:498–506. doi: 10.1165/ajrcmb.19.3.3242. [DOI] [PubMed] [Google Scholar]
- Chu S, Blaisdell CJ, Bamford P, Ferro TJ. Interferon-gamma regulates ClC-2 chloride channel in lung epithelial cells. Biochem. Biophys. Res. Commun. 2004;324:31–39. doi: 10.1016/j.bbrc.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Cormet-Boyaka E, Jablonsky M, Naren AP, Jackson PL, Muccio DD, Kirk KL. Rescuing cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by transcomplementation. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8221–8226. doi: 10.1073/pnas.0400459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Castranova V, Vallyathan V. Comparative cytotoxicity of cadmium and mercury in a human bronchial epithelial cell line (BEAS-2B) and its role in oxidative stress and induction of heat shock protein 70. J. Toxicol. Environ. Health A. 2007;70:852–860. doi: 10.1080/15287390701212695. [DOI] [PubMed] [Google Scholar]
- Harrington MA, Gunderson KL, Kopito RR. Redox reagents and divalent cations alter the kinetics of cystic fibrosis transmembrane conductance regulator channel gating. J. Biol. Chem. 1999;274:27536–27544. doi: 10.1074/jbc.274.39.27536. [DOI] [PubMed] [Google Scholar]
- Howard M, Jiang X, Stolz DB, Hill WG, Johnson JA, Watkins SC, Frizzell RA, Bruton CM, Robbins PD, Weisz OA. Forskolin-induced apical membrane insertion of virally expressed, epitope-tagged CFTR in polarized MDCK cells. Am. J. Physiol. Cell Physiol. 2000;279:C375–C382. doi: 10.1152/ajpcell.2000.279.2.C375. [DOI] [PubMed] [Google Scholar]
- Jin T, Nordberg M, Frech W, Dumont X, Bernard A, Ye TT, Kong Q, Wang Z, Li P, Lundstrom NG, et al. Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad) Biometals. 2002;15:397–410. doi: 10.1023/a:1020229923095. [DOI] [PubMed] [Google Scholar]
- Jones MM, Cherian MG. The search for chelate antagonists for chronic cadmium intoxication. Toxicology. 1990;62:1–25. doi: 10.1016/0300-483x(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol. Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- Kirschvink N, Martin N, Fievez L, Smith N, Marlin D, Gustin P. Airway inflammation in cadmium-exposed rats is associated with pulmonary oxidative stress and emphysema. Free Radic. Res. 2006;40:241–250. doi: 10.1080/10715760500494657. [DOI] [PubMed] [Google Scholar]
- Konstas AA, Koch JP, Tucker SJ, Korbmacher C. Cystic fibrosis transmembrane conductance regulator-dependent up-regulation of Kir1.1 (ROMK) renal K+ channels by the epithelial sodium channel. J. Biol. Chem. 2002;277:25377–25384. doi: 10.1074/jbc.M201925200. [DOI] [PubMed] [Google Scholar]
- Koyuturk M, Yanardag R, Bolkent S, Tunali S. The potential role of combined anti-oxidants against cadmium toxicity on liver of rats. Toxicol. Ind. Health. 2007;23:393–401. doi: 10.1177/0748233707081907. [DOI] [PubMed] [Google Scholar]
- Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- Leikauf GD, McDowell SA, Wesselkamper SC, Hardie WD, Leikauf JE, Korfhagen TR, Prows DR. Acute lung injury: functional genomics and genetic susceptibility. Chest. 2002;121:70S–75S. doi: 10.1378/chest.121.3_suppl.70s. [DOI] [PubMed] [Google Scholar]
- L'Hoste S, Chargui A, Belfodil R, Duranton C, Rubera I, Mograbi B, Poujeol C, Tauc M, Poujeol P. CFTR mediates cadmium-induced apoptosis through modulation of ROS level in mouse proximal tubule cells. Free Radic. Biol. Med. 2009;46:1017–1031. doi: 10.1016/j.freeradbiomed.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li H, Soleimani M, Girijashanker K, Reed JM, He L, Dalton TP, Nebert DW. Cd2+ versus Zn2+ uptake by the ZIP8 HCO3–dependent symporter: kinetics, electrogenicity and trafficking. Biochem. Biophys. Res. Commun. 2008;365:814–820. doi: 10.1016/j.bbrc.2007.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Leng Q, Egan ME, Caplan MJ, Boulpaep EL, Giebisch GH, Hebert SC. CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J. Clin. Invest. 2006;116:797–807. doi: 10.1172/JCI26961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzi ML, Costantini D, Giani M, Appiani AC, Giunta AM. Severe nephropathy in three adolescents with cystic fibrosis. Arch. Dis. Child. 1991;66:1444–1447. doi: 10.1136/adc.66.12.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L, Vangronsveld J, Van Hecke E, Staessen JA. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol. 2006;7:119–126. doi: 10.1016/S1470-2045(06)70545-9. [DOI] [PubMed] [Google Scholar]
- Nordberg GF. Lung cancer and exposure to environmental cadmium. Lancet Oncol. 2006;7:99–101. doi: 10.1016/S1470-2045(06)70548-4. [DOI] [PubMed] [Google Scholar]
- Nzengue Y, Lefebvre E, Cadet J, Favier A, Rachidi W, Steiman R, Guiraud P. Metallothionein expression in HaCaT and C6 cell lines exposed to cadmium. J. Trace Elem. Med. Biol. 2009;23:314–323. doi: 10.1016/j.jtemb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Paakko P, Anttila S, Kokkonen P, Kalliomaki PL. Cadmium in lung tissue as marker for smoking. Lancet. 1988;1:477. doi: 10.1016/s0140-6736(88)91275-5. [DOI] [PubMed] [Google Scholar]
- Paakko P, Kokkonen P, Anttila S, Kalliomaki PL. Cadmium and chromium as markers of smoking in human lung tissue. Environ. Res. 1989;49:197–207. doi: 10.1016/s0013-9351(89)80065-9. [DOI] [PubMed] [Google Scholar]
- Pearson CA, Lamar PC, Prozialeck WC. Effects of cadmium on E-cadherin and VE-cadherin in mouse lung. Life Sci. 2003;72:1303–1320. doi: 10.1016/s0024-3205(02)02379-2. [DOI] [PubMed] [Google Scholar]
- Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L383–L395. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]
- Rennolds J, Boyaka PN, Bellis SL, Cormet-Boyaka E. Low temperature induces the delivery of mature and immature CFTR to the plasma membrane. Biochem. Biophys. Res. Commun. 2008;366:1025–1029. doi: 10.1016/j.bbrc.2007.12.065. [DOI] [PubMed] [Google Scholar]
- Rennolds J, Tower C, Musgrove L, Fan L, Maloney K, Clancy JP, Kirk KL, Sztul E, Cormet-Boyaka E. Cystic fibrosis transmembrane conductance regulator trafficking is mediated by the COPI coat in epithelial cells. J. Biol. Chem. 2008;283:833–839. doi: 10.1074/jbc.M706504200. [DOI] [PubMed] [Google Scholar]
- Soulsby N, Greville H, Coulthard K, Doecke C. Renal dysfunction in cystic fibrosis: is there cause for concern? Pediatr. Pulmonol. 2009;44:947–953. doi: 10.1002/ppul.21086. [DOI] [PubMed] [Google Scholar]
- Takeda AN, Gautschi I, van Bemmelen MX, Schild L. Cadmium trapping in an epithelial sodium channel pore mutant. J. Biol. Chem. 2007;282:31928–31936. doi: 10.1074/jbc.M700733200. [DOI] [PubMed] [Google Scholar]
- Vij N, Mazur S, Zeitlin PL. CFTR is a negative regulator of NFkappaB mediated innate immune response. PLoS One. 2009;4:e4664. doi: 10.1371/journal.pone.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S, Patel S, Kapron CM. The effect of vitamin E exposure on cadmium toxicity in mouse embryo cells in vitro. Toxicology. 2000;142:119–126. doi: 10.1016/s0300-483x(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Welsh MJ. Cigarette smoke inhibition of ion transport in canine tracheal epithelium. J. Clin. Invest. 1983;71:1614–1623. doi: 10.1172/JCI110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Bourgkard E, Paris C. Lung cancer and exposure to metals: the epidemiological evidence. Methods Mol. Biol. 2009;472:139–167. doi: 10.1007/978-1-60327-492-0_6. [DOI] [PubMed] [Google Scholar]