Abstract
Originally identified as an oncogene activated by amplification in squamous cell carcinomas, several lines of evidence now suggest that squamous cell carcinoma-related oncogene (SCCRO; aka DCUN1D1) may play a role in the pathogenesis of a wide range of human cancers including gliomas. SCCRO's oncogenic function is substantiated by its ectopic expression, resulting in transformation of cells in culture and xenograft formation in nude mice. The aim of this study was to assess the in vivo oncogenicity of SCCRO in a murine model. Ubiquitous expression of SCCRO resulted in early embryonic lethality. Because SCCRO overexpression was detected in human gliomas, its in vivo oncogenic activity was assessed in an established murine glioma model. Conditional expression of SCCRO using a replication-competent ASLV long terminal repeat with splice acceptor/nestin-(tumor virus-A) tv-a model system was not sufficient to induce tumor formation in a wild-type genetic background, but tumors formed with increasing frequency and decreasing latency in facilitated background containing Ink4a deletion alone or in combination with PTEN loss. Ectopic expression of SCCRO in glial progenitor cells resulted in lower-grade gliomas in Ink4a-/- mice, whereas its expression in Ink4a-/-/PTEN-/- background produced high-grade glioblastoma-like lesions that were indistinguishable from human tumors. Expression of SCCRO with platelet-derived growth factor-beta (PDGF-β) resulted in an increased proportion of mice forming glioblastoma-like tumors compared with those induced by PDGF-β alone. This work substantiates SCCRO's function as an oncogene by showing its ability to facilitate malignant transformation and carcinogenic progression in vivo and supports a role for SCCRO in the pathogenesis of gliomas and other human cancers.
Introduction
The use of high-throughput, genome-wide screening tools has accelerated the definition of the cancer genome and has identified novel aberrations in many different cancer types. Although informative, the density and complexity of the aberrations identified by screening studies make it difficult to identify those events that are relevant to cancer pathogenesis. As genetic aberrations develop in random fashion and are propagated in a Darwinian manner, those that occur in high frequency and accumulate in cancers from diverse anatomic sites are more likely to harbor cancer-related genes. Losses at 3p and 9p and gains/amplifications at 3q and 11q13 are some examples of loci that are aberrant in multiple cancer types [1–16]. Of particular interest is amplification at 3q because it has been correlated with cancer progression and survival even after controlling for confounding variables [3,13,17]. Moreover, because amplified genomic DNA is inherently unstable, the high prevalence of 3q amplification in lung, head and neck, esophageal, cervical, ovarian, vulvar, and urinary bladder cancers supports its functional significance [1–16]. Several different candidate genes have been identified within the 3q26.3 locus [5,18–20]. However, given the degeneracy of gene expression changes in amplified regions combined with the lack of in vivo confirmation of oncogenic function, the biological significance of individual genes that drive selection for 3q26.3 amplification remains ill defined.
We identified squamous cell carcinoma-related oncogene (SCCRO) within a subpeak of amplification at 3q26.3 using a systematic, positional cloning approach [12,21]. Supporting its candidacy as a target activated by amplification at 3q, SCCRO is amplified and overexpressed in a wide variety of human cancers, and its overexpression is independently associated with an aggressive clinical course [21–24]. Consistent with its activation by amplification, ectopic expression of SCCRO transforms NIH-3T3 cells and HaCaT cells, as evidenced by their ability to form colonies in soft agar and xenografts in nude mice [21]. In addition, we found that knockdown of SCCRO using RNAi or antisense oligonucleotides resulted in apoptosis in tumor cell lines overexpressing SCCRO, suggesting an “oncogene addiction” phenotype (Ganly, et al., unpublished data) [21]. We found that SCCRO binds to neddylation components (CAND1, UBC12, and cullin family of proteins). Further, our biochemical studies show that SCCRO augments neddylation, a posttranslational modification of cullins, which is a regulatory step in cullin RING ligase-mediated protein ubiquitination. Assessment of SCCRO function in cells and model organisms suggests that it is essential for neddylation in vivo. Neddylation, being an established pathway in cancer pathogenesis, supports SCCRO's function as an oncogene [25,26].
Despite the accumulated evidence, whether SCCRO plays a role in human cancer pathogenesis remains to be established. As in vivo tumor formation offers the strongest evidence for the functional importance of an oncogene, we aimed to assess if overexpression of SCCRO results in tumor formation in mice. We found that ubiquitous expression of SCCRO resulted in lethality in mice. As such, we elected to use a conditional somatic gene transfer model using a replication-competent avian leukemia virus (RCAS) vector to deliver SCCRO into murine cells that transgenically express the avian viral receptor (tv-a) [27]. Because we were not successful in delivering the transgene into oral and lung epithelial cells, we screened other tumor types where SCCRO is dysregulated. Because we found that SCCRO is overexpressed in human gliomas, we elected to use an established RCAS/tv-a model of murine gliomagenesis [27,28]. In this model, the nestin promoter was used to drive tv-a expression (Ntv-a) in neuronal and glial progenitor cells in the subventricular zone (SVZ) of mice, the cell of origin for gliomas. Stereotactic injection of RCAS containing the desired transgene in the SVZ allows conditional expression in glioma progenitor cells [29]. We found that SCCRO expression did not induce glioma formation by itself. In contrast, expression of SCCRO promoted gliomagenesis in mice with facilitated genetic backgrounds containing Ink4a deletion alone or in combination with PTEN loss. In addition, coexpression of SCCRO promoted malignant progression of tumors induced by platelet-derived growth factor-β (PDGF-β) in Ntv-a mice. These findings suggest that SCCRO functions as an oncogene in vivo, further validating its candidacy as a target activated by amplification at 3q.
Materials and Methods
Plasmids
The coding sequence of murine SCCRO was cloned into pCDNA3-HA (Invitrogen, Carlsbad, CA), EF1-α promoter containing pCDNA3.1 (Invitrogen), aCMV promoter containing pUSEamp (Upstate Biotechnology, Lake Placid, NY) by standard polymerase chain reaction (PCR) cloning methods. Complementary DNA of SCCRO, polyoma middle T, and GFP were subcloned into RCAS vectors using the Gateway in vitro recombination system (Invitrogen). The RCAS-PDGF-HA and RCAS-Cre expression plasmids were developed as described previously [29]. All constructs were confirmed by DNA sequencing.
Tumor Tissue
Primary brain tumors were collected from patients undergoing surgical resection, after obtaining informed consent and following institutional guidelines. All tumor samples were terminally anonymized after collection. Normal brain cortex was obtained by autopsy of adult human froma commercial source (Analytical Biological Services, Inc, Wilmington, DE).
Immunoblot Analysis and Immunohistochemistry
Antibodies used were Cul1 (Zymed, San Francisco, CA), Cul3 (BD Biosciences, San Jose, CA), tubulin (Calbiochem, San Diego, CA), HA (Covance, Princeton, NJ), Olig2 (Chemicon, Temecula, CA), glial fibrillary acidic protein (GFAP; Bio Genex, San Ramon, CA), MIB1 (Dako, Carpinteria, CA), vimentin (Dako), and S100 (Dako). Antibody against SCCRO was raised in rabbit and used as described earlier [21]. Secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). For immunoblot analysis, the concentrations of antibodies used were according to the manufacturers' specifications. For immunohistochemical analyses, mouse brains were fixed, paraffin-embedded, and processed essentially as described previously [29]. Slides were stained with primary antibody overnight at 4°C. Appropriate secondary biotin-conjugated antibody was applied for 1 hour at room temperature. Peroxidase signal was developed using the ABC kits (Vector Laboratories, Burlingame, CA).
Real-time Reverse Transcription-PCR Analysis
Real-time reverse transcription (RT)-PCR was performed essentially as described earlier [21]. Melt curve analysis was performed after amplification [30]. The acquisition temperature was set 1°C to 2°C below the Tm of the specific PCR product. Quantification of the target gene was in comparison to a reference gene (18S rRNA). PCR primers and conditions are as described earlier [21].
In Vitro Embryonic Stem Cell Differentiation
Culture, transfection, and methods used for in vitro differentiation and analysis of CJ7 ES cells into neurons were performed as previously described [29].
Transgenic Mice
For pronuclear injection, the DNA from plasmid containing SCCRO was sequence-verified, purified, and injected into F2 eggs obtained from matings of (C57BL/6J x CBA/J) F1 mice. Approximately 200 to 250 injected eggs were transferred to pseudopregnant recipients for each round of injection. For the development of chimera, ES cells were stably transfected by electroporation, and antibody was selected. After confirmation of gene expression, transfected ES cells were injected into blastocysts derived from the C57BL/6J inbred mice and transferred into pseudopregnant recipients.
Transgene Expression in tv-a Transgenic Mice
RCAS constructs were propagated in chicken DF1 cells (CRL-12203; ATCC, Manassas, VA), cultured as suggested by ATCC. Only DF1 cells that had been in culture for less than three passages after transfection with RCAS viral complementary DNA were used for infections. Expression of transgenes in DF1 cells was confirmed by immunoblot analysis. The delivery of transgenes by RCAS virus propagated in DF1 cells was confirmed by infecting primary brain cultures derived from Ntv-a mice and by assessing the expression of transgenes in these cells by immunoblot analysis. The function of RCAS-delivered SCCRO in primary brain cultures was assessed by performing neddylation assays as described [31]. Development and validation of mice expressing tv-a under the control of a nestin promoter in wild type and facilitated backgrounds have been described previously [29,32]. DF1 cells transfected with RCAS vectors were trypsinized and suspended in approximately 50 µl of media and were aliquoted for injection. These cells were stereotactically injected into the SVZ of mice as previously described [29]. Before injection, DF1 cells were lysed to confirm transgene expression by immunoblot analysis. The brain from all mice was harvested, fixed in formalin, embedded in paraffin, and subjected to histological and immunohistochemical studies after sectioning as described previously [29]. All specimens were analyzed by an experienced pathologist in a blinded manner. Tumors in mice were considered high grade if they had glioblastoma like features; all others were grouped as lower grade [33].
Statistical Analysis
Statistical analyses were performed using commercially available statistical software packages. Statistical significance was defined as a two-tailed P ≤ .05. Descriptive statistics were used to summarize pertinent study information. Fisher exact test was used for exact nonparametric inferences. Survival curves were generated by the Kaplan-Meier method and were compared using Gehan-Breslow-Wilcoxon test.
Results
Constitutive Expression of SCCRO Results in Embryonic Lethality in Mice
Our prior work suggests overexpression resulting from amplification activates SCCRO's oncogenic function [21]. To determine whether SCCRO functions as an oncogene in vivo, we aimed to develop a mouse model of ectopic SCCRO expression and to assess if this results in spontaneous tumor formation. Despite several attempts, no viable mice were derived from pronuclear injection of embryos with constructs expressing SCCRO under the control of ubiquitously active promoters (β-actin, EF1-α, or CMV). This suggests that global expression of SCCRO results in embryonic lethality. To confirm these results and to determine the timing and cause for lethality, we performed time dam experiments after pronuclear injection of fertilized embryos with a plasmid containing SCCRO under the control of EF1-α promoter. After allowing sufficient time for pronuclear fusion, the derived embryos were implanted into pseudopregnant female mice. The transplanted females were killed when the embryos were approximately E8.5 stage, and the embryos were screened by PCR for the presence of transgene. No viable embryos containing pCMV-SCCRO transgenic construct could be detected, suggesting that those that harbored did not survive. In parallel control experiments, pronuclear injection of embryos with empty vector resulted in grossly normal embryos.
As an alternative approach, we attempted to develop chimeric mice by injecting blastocysts with embryonic stem (ES) cells stably expressing SCCRO under the control of the EF1-α promoter. All experiments were performed using two independent SCCRO-transfected ES cell clones and empty vector-transfected ES cells as controls. SCCRO-transfected ES cells showed higher rates of proliferation but were otherwise phenotypically similar to vector-transfected cells (data not shown). From a total of 120 blastocyst injections, only two mice were born, both of which were less than 10% chimera based on coat color. One of these mice died early from unknown causes, the other died at 3 months of age with multiple tumors in the liver and kidney. Transgenic SCCRO expression in the tumors in these mice was confirmed by PCR (data not shown). However, we were unable to generate additional chimeric mice, limiting the significance of the observed findings. A control group, in which blastocysts were injected with ES cells transfected with an empty vector, yielded normal mice that were 10% to 50% chimera based on coat color and had no obvious developmental defects or tumor formation. Although several possibilities exist, one plausible explanation is that the hyperproliferative SCCRO-transfected ES cells may outgrow native ES cells, which is substantiated by analysis of blastocysts in culture showing a predominance of SCCRO-transfected cells with progressive differentiation (data not shown). To address this issue, we repeated the blastocyst injections with fewer ES cells, as well as an independent SCCRO-transfected ES cell clone, which also did not yield viable litters (data not shown). Combined, these findings suggest that the misexpression of SCCRO is detrimental to murine development, resulting in early embryonic lethality.
Conditional Expression of SCCRO in Epithelial Tissues
Because constitutive expression of SCCRO was lethal, we focused our efforts toward the development of a conditional model of SCCRO overexpression in mice. Given the high prevalence of 3q amplification in squamous cell carcinomas (SCCs) of mucosal origin, we aimed to develop a model for SCCRO overexpression in epithelial progenitor cells that give rise to oral and/or lung SCCs in mice. However, primarily due to the lack of a promoter that drives gene expression exclusively in SCC progenitor cells, there are no well-established transgenic models for noncutaneous SCC in mice. Therefore, we first assessed it in an RCAS/tv-a model to achieve selective gene expression in mucosal keratinocytes, and if that was sufficient for tumorigenesis, RCAS viral constructs containing either GFP or polyoma middle T antigen were propagated in DF1 cells and were injected directly into the oral mucosa of transgenic mice expressing the tv-a receptor under the control of the keratin-5 or β-actin promoter. Despite varying the timing, amount, and the sites of injection, we were unable to successfully infect oral epithelial tissue (data not shown). Although the precise reasons remain to be defined, RCAS virus, which preferentially infects actively proliferating cells, may be limiting in the oral mucosa. In addition, the mucosal epithelial layer is quite thin, making it difficult for the viral inoculum to be delivered to the correct cells. Of note, a poorly differentiated soft tissue tumor did develop in the masseter muscle of two β-actin-tv-a mice injected with RCAS middle T antigen, confirming that the RCAS construct was functional. Similar attempts to develop a RCAS lung SCC model by transthoracic injection of the recombinant virus were also unsuccessful.
SCCRO Is Expressed during Neuronal Development and Is Overexpressed in Human Gliomas
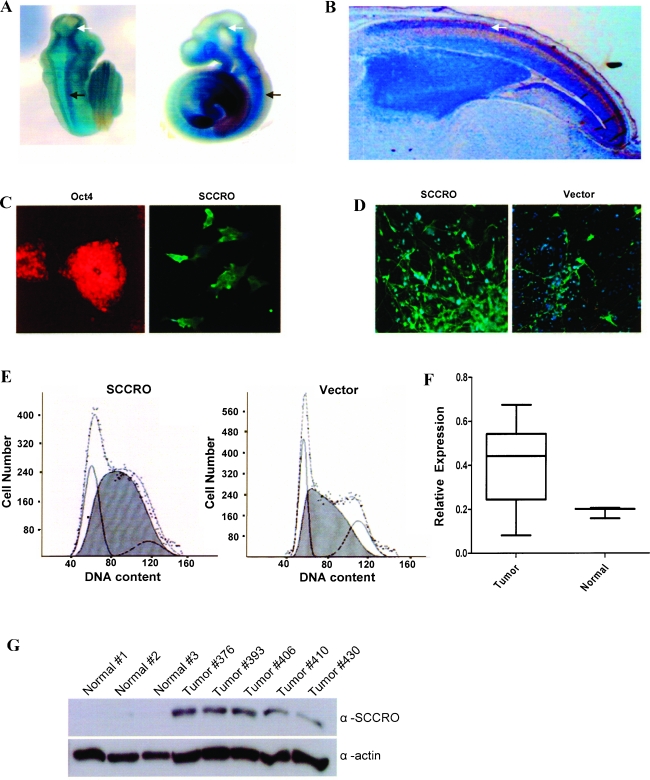
Given our inability to develop a murine SCC model, we focused on the identification of tumor systems with established approaches for transgene expression in which SCCRO plays an oncogenic role. The well-established RCAS-driven glioma model combined with the high incidence of 3q amplification reported in gliomas (64%) led us to investigate SCCRO's function in gliomagenesis [34]. We first aimed to determine whether SCCRO is active in gliomagenesis. Because cancer related genes are often dysregulated developmental genes, we first assessed whether SCCRO function is relevant in neuronal tissues by assessing its role in development using both in vivo murine and in vitro neuronal differentiation model systems. Assessment of transgenic mouse expressing LacZ under the control of the native SCCRO promoter suggested that SCCRO is expressed and developmentally regulated in neuronal tissues (Figure 1A). SCCRO was found to be expressed in the developing mouse forebrain, midbrain, and hindbrain at the early stages of neuronal development (Figure 1B). To substantiate our findings in mice, we monitored the expression of SCCRO during differentiation of ES cells to neurons [35,36]. In contrast to animal models, the differentiation of ES cells allows analysis of the early stages of development and assessment of the role of individual genes that may be involved in the process. ES cells were cocultured and induced to differentiate to both midbrain and hindbrain neuronal fate, and the expression of SCCRO was assessed in different stages of differentiation. Midbrain cell fate was confirmed in each experiment by assessing for tyrosine hydroxylase and Tuj-1 expression. Immunofluorescence using polyclonal antibodies against SCCROshowed pan-cellular distribution of SCCRO throughout the course of differentiation of immature ES cells (Oct4 expressing) to mature neuronal cells (TH and Tuj-1 expressing; Figure 1, C and D). Quantitative analysis by real-time PCR showed that SCCRO expression was highest at day 6 after initiation of ES cell differentiation and declined thereafter. Ectopic expression of SCCRO in stably transfected ES cells resulted in an increase in proliferative activity, as evidenced by an increase in the number of cells in S phase on FACS analysis, bromodeoxyuridine incorporation (data not shown), and neuronal density in controlled culture experiments (Figure 1E). These data suggest that SCCRO is developmentally regulated and that its overexpression can induce proliferation in neural progenitor cells, suggesting that it may impart growth advantages.
Figure 1.
SCCRO is expressed in developing murine neuronal tissue. (A) Transgenic mouse embryo (left-dorsal view; right-lateral view) containing lac-Z under control of SCCRO promoter shows expression of β-galactosidase (lacZ) in developing brain (white arrow) and spinal cord (black arrow) tissue. (B) Sagittal section (x20) showing SCCRO expression in nuclear layer of the neopallial cortex in embryonic (E16) mouse brain (arrow). (C) Expression of SCCRO (right) in Oct4 (left)-expressing immature ES cells. (D) SCCRO-transfected ES cells show increased neuron density (green) in controlled culture experiments. (E) ES cells transfected with SCCRO show increased number of cells in S phase relative to vector-transfected. (F) SCCRO mRNA expression in human glioblastomas (n = 12) and normal brain tissue (n = 3) by RT-PCR. (G) Immunoblot for SCCRO on normal brain tissue and human glioblastomas overexpressing SCCRO mRNA as determined by RT-PCR.
The high incidence of 3q amplification reported in human gliomas (64%) led us to investigate if SCCRO is a target of 3q amplification in these tumors. We assessed its messenger RNA (mRNA) and protein levels in human glioblastomas. Overexpression of SCCRO mRNA and protein was seen in primary gliomas relative to normal brain tissue as detected by real-time RT-PCR and Western blot analyses (Figure 1, F and G). In addition, immunohistochemical analyses of tissue microarrays showed that SCCRO is overexpressed (> 2+ staining) in anaplastic oligodendroma (13/42 cases, 31%), hemangioblastoma (4/12 cases, 33%), and glioblastoma (2/25 cases, 8%; data not shown). Combined, these data support a role for SCCRO in human gliomagenesis.
Expression of SCCRO in Glial Cells Results in Oligodendroma in Facilitated Genetic Backgrounds
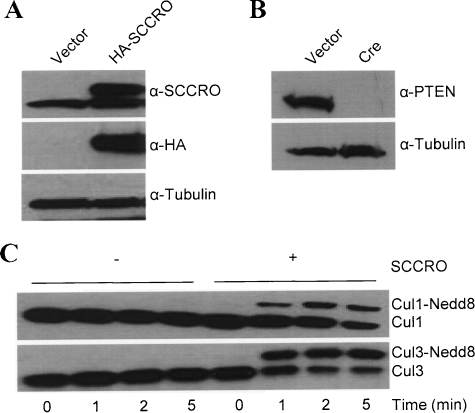
To substantiate its role in gliomagenesis, we used the RCAS/tv-a system to deliver and express SCCRO in the glial progenitor cells in the SVZ of transgenic mice expressing tv-a under the control of a brain-specific nestin promoter (Ntv-a) [37]. As activation of SCCRO may be a late event in malignant progression, we used a facilitated genetic background of Ink4a-/- with and without PTEN using Ink4a/Arf-/-/loxPPTENloxP mice. RCAS-HA-SCCRO or RCAS-Cre recombinase was propagated in DF1 cells. The ability of the RCAS virus to infect the primary brain cells fromNtv-a transgenic mice and express the transgene (SCCRO and Cre) was confirmed by Western blot after infection of primary brain cultures from Ntv-a transgenic mice (Figure 2A). As expected, expression of Cre resulted in loss of PTEN (Figure 2B). To confirm that SCCRO retained its known biochemical activity in primary brain cells, we performed an in vitro neddylation assay essentially as described earlier [31,38,39]. SCCRO promotes Cul1 and Cul3 neddylation, thereby establishing brain cells as a model to study its function (Figure 2C).
Figure 2.
Transgenic expression of SCCRO using the RCAS system. (A) Immunoblot probed for SCCRO and HA showing the levels of HA-SCCRO (top panel; upper band) and HA (middle panel) in primary brain cell culture extracts from Ntv-a/Ink4a/Arf-/-/loxPPTENloxP mice infected with RCAS-HA-SCCRO virus. (B) Extracts of same cells transfected with Cre recombinase results in loss of PTEN expression. (C) SCCRO functions to augment cullin neddylation in Ntv-a Ink4a/Arf-/- cell lysate. In vitro neddylation reaction containing primary brain cell culture extracts from Ntv-a Ink4a/Arf-/-. Mice as a source of cullins were incubated with neddylation components (E1, E2, ATP, and Nedd8) for the indicated time in the absence or presence of recombinant SCCRO. Immunoblot of the reaction mixture probed for Cul1 (top) and Cul3 (bottom).
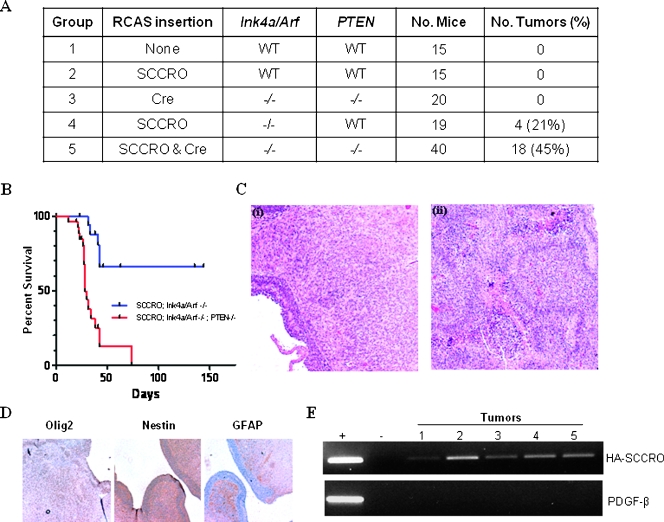
The oncogenic activity of SCCRO was tested in neonatal mice expressing Ntv-a, which were divided into five experimental groups (Figure 3A). RCAS virus containing SCCRO and/or Cre was stereotactically introduced into the SVZ of these mice. Ntv-a mice in groups 1 and 2 were infected with RCAS vector or RCAS-SCCRO, respectively. Ntv-a/Ink4a/Arf-/-/loxPPTENloxP mice in groups 3, 4, and 5 were infected with RCAS-Cre, RCAS-SCCRO, or both, respectively. Mice were killed when they showed evidence of abnormal behavior, cephalomegaly, seizures, or at 6 months if they remained asymptomatic. As expected, none of the mice injected with DF1 cells expressing RCAS alone (group 1) or RCAS-Cre (group 3) developed tumors. In addition, mice injected with RCAS-SCCRO alone (group 2) did not develop tumors by 6 months. Confirming that a longer latency was not required for SCCRO to promote tumorigenesis, an independent group of mice injected with RCAS-SCCRO (n = 17) also did not develop tumors even at 12 months. Tumors were seen in Ntv-a/Ink4a/Arf-/-/loxP PTENloxP mice injected with RCAS-SCCRO or RCAS-SCCRO with RCAS-Cre (groups 4 and 5, respectively). Overall, 4 (21%) of 19 mice injected with RCAS-SCCRO in Ntv-a/Ink4a/Arf-/-/loxPPTENloxP background and 18 (45%) of 40 injected with RCAS-SCCROand RCAS-Cre (in Ink4a/Arf-/-/loxPPTENloxP background) developed tumors, suggesting that SCCRO can induce tumor formation in a facilitated background (χ2 test, P < .001). The latency for SCCRO-induced tumor formation was shorter in Ink4a/Arf-/-/PTEN-/- background (Figure 3B). Tumors in Ink4a/Arf-/- were low grade (Figure 3C), with a lower proliferative index based on lower mitotic rates and staining for MIB1 (<20% Ink4a/Arf-/- vs 50%–70% for Ntv-a/Ink4a/Arf -/-/loxPPTENloxP background). In contrast, tumors that developed in the Ink4a/Arf -/-/PTEN-/- background were high grade with palisading necrosis that was histopathologically indistinguishable from human glioblastomas (Figure 3C). Immunophenotyping showed that all tumors in these experiments were oligodendromas because they expressed Olig2 but not GFAP, which is expressed in astrocytes (Figure 3D). As expected, no tumors developed in mice lacking the Ntv-a transgene regardless of the virus or combination of viruses with which they were infected (data not shown). These observations suggest that, although it is insufficient by itself, SCCRO promotes gliomagenesis in a facilitated genetic background.
Figure 3.
SCCRO overexpression promotes glioma formation in a facilitated genetic background. RCAS-SCCRO was stereotactically injected into the SVZ of neonatal mice of various genetic backgrounds. (A) SCCRO induced tumor formation only in a facilitated genetic background (groups 4 and 5; Fisher exact test, P < .001). (B) Disease-specific survival of Ntv-a mice. Loss of tumor suppressor PTEN is associated with shorter latency (Gehan-Breslow-Wilcoxon test, P < .001). (C) Representative hematoxylin and eosin-stained sections showing the histopathology of RCAS-SCCRO-induced tumor in two different genetic backgrounds: (i) Ntv-a Ink4a/Arf-/- mouse injected with RCAS-SCCRO produces low-grade tumors and exhibits a subependymal location. (ii) Ntv-a Ink4a/Arf-/-, PTEN-/- mouse injected with RCAS-SCCRO results in high-grade tumor that diffusely invades brain parenchyma and exhibits increased vascularity. (D) Immunohistochemical analysis showing expression of Olig2 but not GFAP or nestin in brain tumor sections. (E) Agarose gel showing the PCR products of DNA extracted from RCAS-HA-SCCRO-injected brain tissues using primers specific for HA-SCCRO (top) or PDGF-β (bottom), an unrelated gene as a negative control (lanes 1–5). (+) PCR products of the plasmid template containing HA-SCCRO (top) or PDGF-β (bottom) as positive control. (-) PCRs without the template as negative control.
To determine whether SCCRO-mediated glioma formation is cell autonomous, we assessed for nestin expression in tumors, which is a marker for cells of glial origin. Immunohistochemical analyses of representative tumors showed strong nestin expression in all samples (n = 15), reflecting their glial lineage (Figure 3D). To establish a cause-and-effect relationship, microdissected tumor samples, but not adjacent normal brain tissue, showed the presence of RCAS-SCCRO as determined by PCR analyses (Figure 3E, upper panel). As a negative control, we assessed for the presence of an unrelated oncogene, hPDGF-β, which was not detected in any of the tumor samples tested (Figure 3E, lower panel). These findings suggest that SCCRO functions in a cell autonomous manner to promote oligodendromas in mice.
SCCRO Promotes Malignant Progression of PDGF-Dependent Gliomas
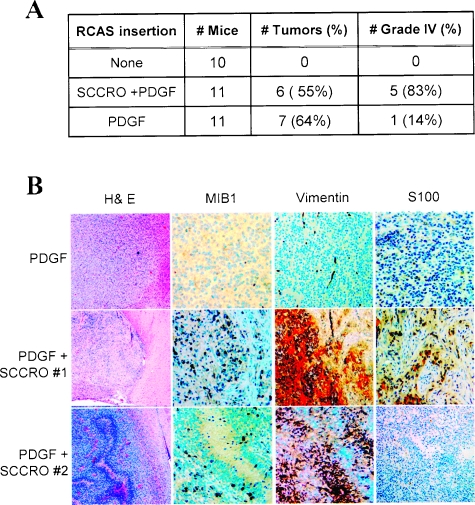
PDGF-β is overexpressed and implicated in multiple tumor types, including gliomas [40]. Previous studies show that expression of PDGF-β alone in nestin-positive progenitors is sufficient to induce glioma formation in 60% of mice by 12 weeks [29]. Moreover, PDGF-β induces tumor formation in a dose-dependent manner. Whereas low levels of expression are associated with fewer tumors and low-grade histological diagnosis, deletion of the inhibitory elements in the 5′UTR of PDGF-β mRNA, which results in higher protein levels, results in the formation of higher-grade gliomas and decreased tumor latency in mice [41]. Moreover, low-level expression of PDGF-β in facilitated genetic backgrounds also results in the development of high-grade gliomas, recapitulating the genetic complexity of human glioblastomas [42]. To assess if SCCRO contributes to the progression of low-grade gliomas to glioblastomas, we assessed the combined effects of RCAS-SCCRO and RCAS-PDGF-β expression (low-level expression with a construct containing the entire 5′UTR) in Ntv-a mice. Although the latency and frequency of tumor formation were not significantly changed, coexpression of SCCRO and PDGF-β was associated with high-grade tumors, with 66% (5/6) of these mice having glioblastoma-like histological diagnosis in contrast to only 14% (1/7) of mice injected with PDGF-β alone (Fisher exact test, P = .03; Figure 4A). Consistent with this observation, tumors in mice resulting from coexpression of SCCRO and PDGF-β had increased MIB1 expression, reflecting their higher rate of mitoses observed in these tumors (Figure 4B). Furthermore, tumors in these mice were associated with unique immunohistochemical features. Expression of vimentin (4/6) and loss of S100 expression (3/6) were observed only in tumors from mice infected with both RCAS-SCCRO and RCAS-PDGF-β (Figure 4B). Loss of neural crest markers (S100) and expression of mesenchymal markers (vimentin) are consistent with dedifferentiated histopathological findings in tumors resulting from the expression of SCCRO and PDGF-β. These findings suggest that SCCRO contributes rather than being the primary cause of the progression to malignant glioblastoma.
Figure 4.
PDGF-β cooperates with SCCRO to produce poorly differentiated glioblastoma. (A) Although no significant change in overall incidence was observed, tumors induced by RCAS-PDGF-β alone were primarily low grade, whereas those induced by in combination with RCAS-SCCRO were high grade (Fisher exact test, P = .03). (B) Brain sections of mice infected with RCAS-SCCRO and RCAS-PDGF-β show a distinct immunohistochemical profile with increased MIB1 expression and vimentin expression relative to those infected with RCAS-PDGF-β alone. Loss of S100 expression was seen in RCAS-SCCRO- and RCAS-PDGF-β-induced gliomas.
Discussion
Amplification at 3q, a common event in many human cancers, is associated with progression to invasive cancer and survival, even after controlling for confounding variables [12,14]. Given its high frequency and clinical significance, identification of gene targets of 3q amplification has been a focus of considerable interest. Because the minimal common amplified region at 3q is large, we used a systematic positional cloning approach to identify a subpeak at 3q26.3 that contained SCCRO [12,14]. Overexpression of SCCRO in benign transformed cells (NIH-3T3 and HaCaT) resulted in malignant transformation based on in vitro and xenograft assays in nude mice [21]. However, results from these experiments are not sufficient to implicate SCCRO in cancer pathogenesis. Because demonstration of in vivo oncogenic activity offers the strongest evidence that a gene plays a role in cancer pathogenesis, we attempted to develop transgenic mice expressing SCCRO under the control of constitutive promoters to assess its oncogenic function. We found that overexpression of SCCRO was lethal, likely at the preimplantation stage. As such, we could not make any further conclusions about the cause for lethality in these mice. These results are consistent with the known function of SCCRO because dysregulation of neddylation pathway component is associated with detrimental developmental effects.
Given the lethality observed in transgenic animals, we next elected to develop a conditional model to assess the effects of SCCRO overexpression. Because 3q amplification has a predilection for SCC of mucosal origin, our preference was to develop a model that allows overexpression of SCCRO in epithelial cells. Considering no models were available that allowed conditional expression of SCCRO in progenitor cells that give rise to mucosal SCCs, we aimed to develop a novel model using the RCAS/tv-a system. Despite trying many conditions, we were not able to express SCCRO in sufficient levels in lung and oral mucosa of mice. As such, we elected to look for other models that would allow assessment of in vivo oncogenicity of SCCRO.
Like most cancers, glioma is a genetically complex disease with multiple pathways dysregulated. Gene amplification events are a key mechanism for oncogene activation in human gliomas. These include EGFR (7p12), PDGFRA (4q12), CDK4, and MDM2 (12q13–15) [43]. Genomic screening studies have identified many other recurrent aberrations in human gliomas [44]. Among these is 3q amplification, which is present in as many as 64%of gliomas [34]. Because SCCRO plays a role in neuronal development, its overexpression results in increased proliferation in neuronal progenitors and overexpression in human gliomas, it represents a putative target of 3q amplification in human gliomas. As such, we elected to test SCCRO's in vivo oncogenic activity in a RCAS/tv-a murine glioma model. In this model, the nestin promoter (Ntv-a) is used to drive tv-a expression in neuronal and glial progenitor cells in the SVZ, the cell of origin for gliomas. We found that RCAS-driven ectopic expression of SCCRO was not sufficient to induce gliomagenesis on its own. This is not surprising given that 3q amplification is a late event in cancer pathogenesis, by which time many other genetic aberrations have already accumulated in the cell. To try and recapitulate the temporal sequence and genetic background in which 3q amplification may occur during gliomagenesis, we assessed the effects of SCCRO expression in mice with a facilitated genetic background. We deleted Ink4a/Arf-/- and PTEN in NTv-a in mice to facilitate cancer progression because these genes are known to be mutated (≥30%) in human gliomas [45]. Whereas mutation of these genes alone or in combination was not sufficient to induce tumor formation, 21% of mice (n = 19) injected with RCAS-SCCRO in Ink4a/Arf -/- background developed tumors. Expression of SCCRO in mice deficient in both PTEN and Ink4a/Arf resulted in a shorter latency, higher frequency 45% (n = 40), and higher grade of tumors in mice. Similar results have been reported for EGFR, which formed gliomas only when these tumor suppressor genes are mutated [46]. These findings suggest either that SCCRO is weakly oncogenic or that it plays a supportive rather than a direct role in cancer pathogenesis. These findings are consistent with observations in oral SCC where amplification at 3q and the associated activation of SCCRO is a late event in carcinogenesis, occurring in the transition from in situ to invasive cancer [14]. In the time frame during which 3q amplification occurs, multiple genetic aberrations have already accumulated in the fated cell. Combined, these data implicate SCCRO as a candidate protooncogene that is activated by amplification in human cancers.
Unlike SCCRO or EGFR, ectopic expression of PDGF-β alone is sufficient to promote oligodendroglioma in neural progenitor cells. However, PDGF-β expression in an Ink4a/Arf -/- background reduced tumor latency and promoted progression toward a less-differentiated phenotype [29]. Further substantiating its putative role in human gliomagenesis, coexpression of SCCRO with PDGF-β was associated with high-grade tumor. Consistent with this observation, these tumors had increased MIB1 expression and acquired unique immunohistochemical features, with vimentin expression and loss of S100 expression only seen in SCCRO associated tumors. The higher grade and increased mitotic rate, combined with acquisition of unique immunohistochemical features, suggest that SCCRO contributes to malignant progression of PDGF-β-induced tumors. These findings suggest that SCCRO supports malignant progression in gliomagenesis. This may explain the observation in oral and lung cancers where overexpression of SCCRO is associated with a more aggressive clinical course and worse outcome.
Although the precise oncogenic mechanisms remain to be defined, recent studies have shown that SCCRO promotes neddylation of cullins, a regulatory step in protein ubiquitination [31]. Ubiquitination is the principal process that controls proteosomal degradation and, as such directly, affects diverse cellular functions such as cell cycle regulation, signaling, and replication [47,48]. Given its function in neddylation, SCCRO can affect the function of a wide variety of proteins by regulating their ubiquitination, a process that seems essential in carcinogenesis. As an example, c-myc expression is not sufficient to induce tumor formation in mice. Mutations that block ubiquitination of c-myc are more oncogenic [49]. The high frequency and diversity of tumors in which SCCROis activated raises the possibility that dysfunction in neddylation and, in turn, ubiquitination may be a key component in carcinogenesis.
Acknowledgments
The authors thank Eric Holland and Andrew Koff for their guidance, Anne Conway and Peter Meisel for technical assistance, and Nancy Bennett for her outstanding editorial assistance.
Abbreviations
- GFAP
glial fibrillary acidic protein
- RCAS
replication-competent ASLV long terminal repeat with splice acceptor
- SCCRO
squamous cell carcinoma-related oncogene
- tv-a
tumor virus-A
- SVZ
subventricular zone
Footnotes
This work was supported in part by grants from George H.A. Clowes, Jr., MD, FACS, Memorial Research Career Development Award from the American College of Surgeons, Falcone Fund, and the Clinical Innovator Award, Flight Attendant Medical Research Institute (to B.S.).
References
- 1.Balsara BR, Sonoda G, du Manoir S, Siegfried JM, Gabrielson E, Testa JR. Comparative genomic hybridization analysis detects frequent, often high-level, overrepresentation of DNA sequences at 3q, 5p, 7p, and 8q in human non-small cell lung carcinomas. Cancer Res. 1997;57:2116–2120. [PubMed] [Google Scholar]
- 2.Bjorkqvist AM, Husgafvel-Pursiainen K, Anttila S, Karjalainen A, Tammilehto L, Mattson K, Vainio H, Knuutila S. DNA gains in 3q occur frequently in squamous cell carcinoma of the lung, but not in adenocarcinoma. Genes Chromosomes Cancer. 1998;22:79–82. [PubMed] [Google Scholar]
- 3.Bockmuhl U, Schluns K, Kuchler I, Petersen S, Petersen I. Genetic imbalances with impact on survival in head and neck cancer patients. Am J Pathol. 2000;157:369–375. doi: 10.1016/S0002-9440(10)64549-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brieger J, Jacob R, Riazimand HS, Essig E, Heinrich UR, Bittinger F, Mann WJ. Chromosomal aberrations in premalignant and malignant squamous epithelium. Cancer Genet Cytogenet. 2003;144:148–155. doi: 10.1016/s0165-4608(02)00936-6. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto Y, Oga A, Kawauchi S, Furuya T, Shimizu N, Nakano T, Imate Y, Yamashita H, Sasaki K. Amplification of 3q26 approximately qter correlates with tumor progression in head and neck squamous cell carcinomas. Cancer Genet Cytogenet. 2001;129:52–56. doi: 10.1016/s0165-4608(01)00425-3. [DOI] [PubMed] [Google Scholar]
- 6.Heselmeyer K, Macville M, Schrock E, Blegen H, Hellstrom AC, Shah K, Auer G, Ried T. Advanced-stage cervical carcinomas are defined by a recurrent pattern of chromosomal aberrations revealing high genetic instability and a consistent gain of chromosome arm 3q. Genes Chromosomes Cancer. 1997;19:233–240. [PubMed] [Google Scholar]
- 7.Heselmeyer K, Schrock E, du Manoir S, Blegen H, Shah K, Steinbeck R, Auer G, Ried T. Gain of chromosome 3q defines the transition from severe dysplasia to invasive carcinoma of the uterine cervix. Proc Natl Acad Sci USA. 1996;93:479–484. doi: 10.1073/pnas.93.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kettunen E, el-Rifai W, Bjorkqvist AM, Wolff H, Karjalainen A, Anttila S, Mattson K, Husgafvel-Pursiainen K, Knuutila S. A broad amplification pattern at 3q in squamous cell lung cancer—a fluorescence in situ hybridization study. Cancer Genet Cytogenet. 2000;117:66–70. doi: 10.1016/s0165-4608(99)00146-6. [DOI] [PubMed] [Google Scholar]
- 9.Mayama T, Fukushige S, Shineha R, Nishihira T, Satomi S, Horii A. Frequent loss of copy number on the long arm of chromosome 21 in human esophageal squamous cell carcinoma. Int J Oncol. 2000;17:245–252. [PubMed] [Google Scholar]
- 10.Riazimand SH, Welkoborsky HJ, Bernauer HS, Jacob R, Mann WJ. Investigations for fine mapping of amplifications in chromosome 3q26.3-28 frequently occurring in squamous cell carcinomas of the head and neck. Oncology. 2002;63:385–392. doi: 10.1159/000066220. [DOI] [PubMed] [Google Scholar]
- 11.Shinomiya T, Mori T, Ariyama Y, Sakabe T, Fukuda Y, Murakami Y, Nakamura Y, Inazawa J. Comparative genomic hybridization of squamous cell carcinoma of the esophagus: the possible involvement of the DPI gene in the 13q34 amplicon. Genes Chromosomes Cancer. 1999;24:337–344. [PubMed] [Google Scholar]
- 12.Singh B, Gogineni SK, Sacks PG, Shaha AR, Shah JP, Stoffel A, Rao PH. Molecular cytogenetic characterization of head and neck squamous cell carcinoma and refinement of 3q amplification. Cancer Res. 2001;61:4506–4513. [PubMed] [Google Scholar]
- 13.Singh B, Reddy PG, Goberdhan A, Walsh C, Dao S, Ngai I, Chou TC, O-charoenrat P, Levine AJ, Rao PH, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16:984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B, Stoffel A, Gogineni S, Poluri A, Pfister DG, Shaha AR, Pathak A, Bosl G, Cordon-Cardo C, Shah JP, et al. Amplification of the 3q26.3 locus is associated with progression to invasive cancer and is a negative prognostic factor in head and neck squamous cell carcinomas. Am J Pathol. 2002;161:365–371. doi: 10.1016/S0002-9440(10)64191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh B, Wreesmann VB, Pfister D, Poluri A, Shaha AR, Kraus D, Shah JP, Rao PH. Chromosomal aberrations in patients with head and neck squamous cell carcinoma do not vary based on severity of tobacco/alcohol exposure. BMC Genet. 2002;3:22. doi: 10.1186/1471-2156-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugita M, Tanaka N, Davidson S, Sekiya S, Varella-Garcia M, West J, Drabkin HA, Gemmill RM. Molecular definition of a small amplification domain within 3q26 in tumors of cervix, ovary, and lung. CancerGenet Cytogenet. 2000;117:9–18. doi: 10.1016/s0165-4608(99)00135-1. [DOI] [PubMed] [Google Scholar]
- 17.Wreesmann VB, Singh B. Chromosomal aberrations in squamous cell carcinomas of the upper aerodigestive tract: biologic insights and clinical opportunities. J Oral Pathol Med. 2005;34:449–459. doi: 10.1111/j.1600-0714.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 18.Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, Yang JM. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res. 2001;61:3806–3809. [PubMed] [Google Scholar]
- 19.Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000;97:5462–5467. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 21.Sarkaria I, O-charoenrat P, Talbot SG, Reddy PG, Ngai I, Maghami E, Patel KN, Lee B, Yonekawa Y, Dudas M, et al. Squamous cell carcinoma related oncogene/DCUN1D1 is highly conserved and activated by amplification in squamous cell carcinomas. Cancer Res. 2006;66:9437–9444. doi: 10.1158/0008-5472.CAN-06-2074. [DOI] [PubMed] [Google Scholar]
- 22.Estilo CL, O-charoenrat P, Ngai I, Patel SG, Reddy PG, Dao S, Shaha AR, Kraus DH, Boyle JO, Wong RJ, et al. The role of novel oncogenes squamous cell carcinoma-related oncogene and phosphatidylinositol 3-kinase p110α in squamous cell carcinoma of the oral tongue. Clin Cancer Res. 2003;9:2300–2306. [PubMed] [Google Scholar]
- 23.O-charoenrat P, Sarkaria I, Talbot SG, Reddy P, Dao S, Ngai I, Shaha A, Kraus D, Shah J, Rusch V, et al. SCCRO (DCUN1D1) induces extracellular matrix invasion by activating matrix metalloproteinase 2. Clin Cancer Res. 2008;14:6780–6789. doi: 10.1158/1078-0432.CCR-08-0719. [DOI] [PubMed] [Google Scholar]
- 24.Talbot SG, O-charoenrat P, Sarkaria IS, Ghossein R, Reddy P, Ngai I, Cordeiro CN, Wong RJ, Kris MG, Rusch VW, et al. Squamous cell carcinoma related oncogene regulates angiogenesis through vascular endothelial growth factor-A. Ann Surg Oncol. 2004;11:530–534. doi: 10.1245/ASO.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Guardavaccaro D, Pagano M. Oncogenic aberrations of cullin-dependent ubiquitin ligases. Oncogene. 2004;23:2037–2049. doi: 10.1038/sj.onc.1207413. [DOI] [PubMed] [Google Scholar]
- 26.Sufan RI, Ohh M. Role of the NEDD8 modification of Cul2 in the sequential activation of ECV complex. Neoplasia. 2006;8:956–963. doi: 10.1593/neo.06520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orsulic S. An RCAS-TVA-based approach to designer mouse models. Mamm Genome. 2002;13:543–547. doi: 10.1007/s00335-002-4003-4. [DOI] [PubMed] [Google Scholar]
- 28.Witte HT, Jeibmann A, Klambt C, Paulus W. Modeling glioma growth and invasion in Drosophila melanogaster. Neoplasia. 2009;11:882–888. doi: 10.1593/neo.09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison TB, Weis JJ, Wittwer CT. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24:954–958. 960, 962. [PubMed] [Google Scholar]
- 31.Kim AY, Bommelje CC, Lee BE, Yonekawa Y, Choi L, Morris LG, Huang G, Kaufman A, Ryan RJ, Hao B, et al. SCCRO (DCUN1D1) is an essential component of the E3 complex for neddylation. J Biol Chem. 2008;283:33211–33220. doi: 10.1074/jbc.M804440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland EC. A mouse model for glioma: biology, pathology, and therapeutic opportunities. Toxicol Pathol. 2000;28:171–177. doi: 10.1177/019262330002800122. [DOI] [PubMed] [Google Scholar]
- 33.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui AB, Lo KW, Yin XL, Poon WS, Ng HK. Detection of multiple gene amplifications in glioblastoma multiforme using array-based comparative genomic hybridization. Lab Invest. 2001;81:717–723. doi: 10.1038/labinvest.3780280. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 36.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 38.Kurz T, Chou YC, Willems AR, Meyer-Schaller N, Hecht ML, Tyers M, Peter M, Sicheri F. Dcn1 functions as a scaffold-type E3 ligase for cullin neddylation. Mol Cell. 2008;29:23–35. doi: 10.1016/j.molcel.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Kurz T, Ozlu N, Rudolf F, O'Rourke SM, Luke B, Hofmann K, Hyman AA, Bowerman B, Peter M. The conserved protein DCN-1/Dcn1p is required for cullin neddylation in C. elegans and S. cerevisiae. Nature. 2005;435:1257–1261. doi: 10.1038/nature03662. [DOI] [PubMed] [Google Scholar]
- 40.Shih AH, Holland EC. Platelet-derived growth factor, (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139–147. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64:4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Yeh N, Zhu XH, Leversha M, Cordon-Cardo C, Ghossein R, Singh B, Holland E, Koff A. Somatic cell type specific gene transfer reveals a tumor-promoting function for p21(Waf1/Cip1) EMBO J. 2007;26:4683–4693. doi: 10.1038/sj.emboj.7601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruano Y, Mollejo M, Ribalta T, Fiano C, Camacho FI, Gomez E, de Lope AR, Hernandez-Moneo JL, Martinez P, Melendez B. Identification of novel candidate target genes in amplicons of glioblastoma multiforme tumors detected by expression and CGH microarray profiling. Mol Cancer. 2006;5:39. doi: 10.1186/1476-4598-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeMercier M, Fortin S, Mathieu V, Roland I, Spiegl-Kreinecker S, Haibe-Kains B, Bontempi G, Decaestecker C, Berger W, Lefranc F, et al. Galectin 1 proangiogenic and promigratory effects in the Hs683 oligodendrogliomamodel are partly mediated through the control of BEX2 expression. Neoplasia. 2009;11:485–496. doi: 10.1593/neo.81526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duerr EM, Rollbrocker B, Hayashi Y, Peters N, Meyer-Puttlitz B, Louis DN, Schramm J, Wiestler OD, Parsons R, Eng C, et al. PTEN mutations in gliomas and glioneuronal tumors. Oncogene. 1998;16:2259–2264. doi: 10.1038/sj.onc.1201756. [DOI] [PubMed] [Google Scholar]
- 46.Zhu H, Acquaviva J, Ramachandran P, Boskovitz A, Woolfenden S, Pfannl R, Bronson RT, Chen JW, Weissleder R, Housman DE, et al. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci USA. 2009;106:2712–2716. doi: 10.1073/pnas.0813314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 48.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 49.Ciechanover A, DiGiuseppe JA, Bercovich B, Orian A, Richter JD, Schwartz AL, Brodeur GM. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci USA. 1991;88:139–143. doi: 10.1073/pnas.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]