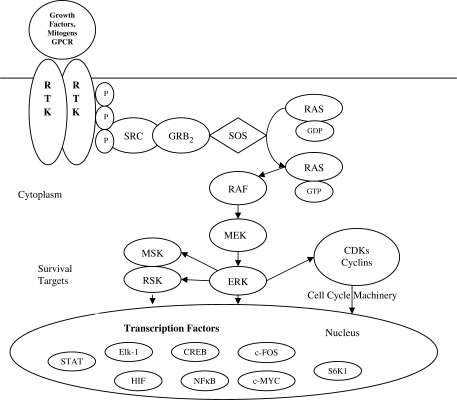
Figure 1.
Receptor-linked tyrosine kinases such as the epidermal growth factor receptor (EGFR) are activated by extracellular ligands. Binding of epidermal growth factor (EGF) to the EGFR activates the tyrosine kinase activity of the cytoplasmic domain of the receptor. The EGFR becomes phosphorylated on tyrosines. Docking proteins such as GRB2 contain SH2 domains that bind to the phosphotyrosines of the activated receptor. BRB2 binds to the guanine nucleotide exchange factor SOS by way of and SH3 domain of GRB2. When the GRB2–SOS complex docks to phosphorylated EGFR, SOS becomes activated. Activated SOS promotes the removal of GDP from Ras. Ras can then bind GTP and become active. Activated Ras activates the protein kinase activity of RAF kinase. RAF kinase phosphorylates and activates MEK, which in turn phosphorylates and activates ERK (extracellular signal-regulated kinase) or mitogen-activated protein kinase (MAPK). The MAPK/ERK cascade may signal survival, cell growth and cell cycle progression. Its effect on RSK (ribosomal S6 kinase) and MSK (mitogen and stress-activated protein kinase) regulate the translational machinery, influencing cell growth and cell division.