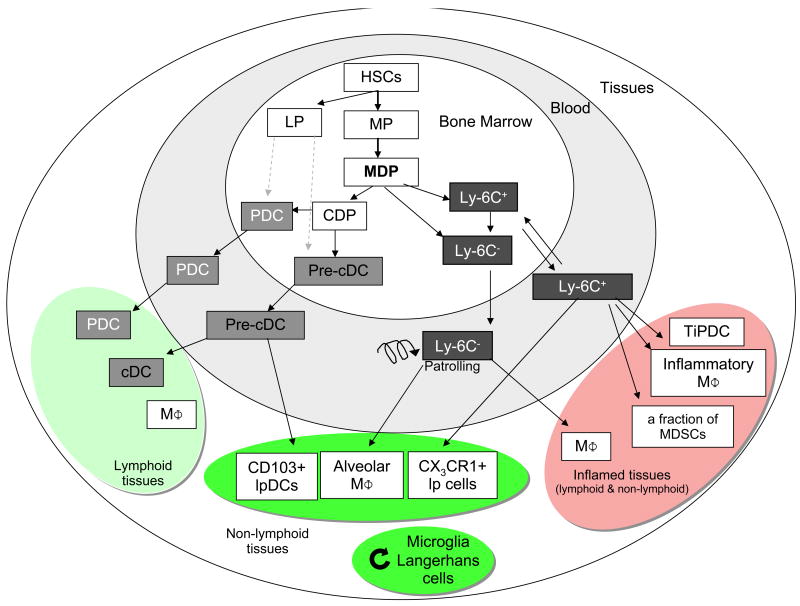
Fig. 2.
Differentiation of DCs and macrophages in mice. In the bone marrow, hematopoietic stem cells (HSC) produce myeloid (MP) and lymphoid (LP) committed precursors. MP give rise to monocyte/macrophages and DC precursors (MDP). MDP give rise to monocytes, and to common DC precursor (CDP). Two monocyte subsets, Ly-6C+ and Ly-6C- leave the bone marrow to enter the blood. CDP give rise to pre-classical dendritic cells (pre-cDC) and plasmacytoid dendritic cells (PDC). Pre-cDC circulate in blood and enter lymphoid tissue, where they give rise to CD8α+ and CD8α- cDCs, and non-lymphoid tissues, where they may give rise to CD103+ lamina propria DC (lpDC). Under homeostatic conditions, Ly-6C- monocytes may contribute to alveolar macrophages (MΦ) and Ly-6C+ monocytes can become CX3CR1+ lpDCs in non-lymphoid tissues. During inflammation, Ly-6C+ monocytes give rise to monocyte-derived DCs, e.g. TNF and iNOS-producing dendritic cells (TipDC), inflammatory macrophages, and may contribute to myeloid-derived suppressor cells (MDSC) associated with tumors. They are also suspected to contribute to microglia and Langerhans cells in selected experimental conditions. Microglia and Langerhans cells can renew independently from the bone marrow (curved arrow). HSC can also leave their bone marrow niche and enter peripheral tissues, where they differentiate to myeloid cells during inflammation. It is unclear at this time if LP contribute significantly to PDC and cDCs (dashed arrow).