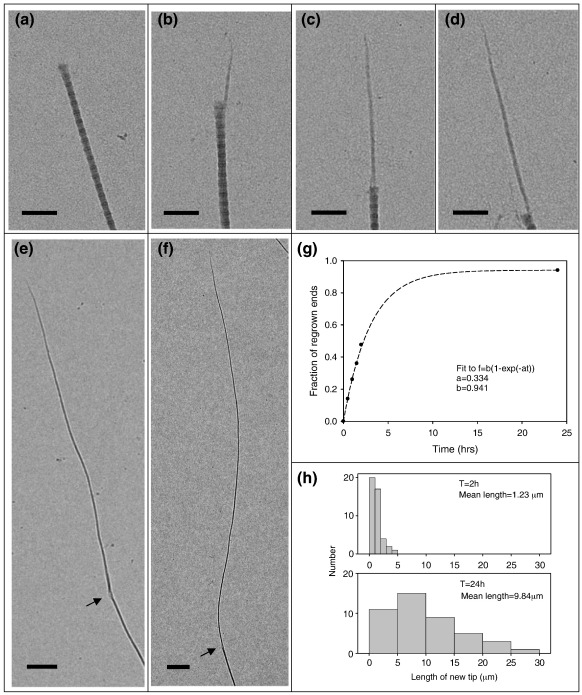
Fig. 1.
TEM of fibril seed growth after increasing incubation times in collagen solution. Fibril seeds were released from 13-day chick embryonic metatarsal tendon by crushing at liquid nitrogen temperature and then dispersing in “fibril dispersion” buffer [50 mM Tris–HCl, 50 mM EDTA (ethylenediaminetetraacetic acid), 150 mM NaCl, and 100 mM sucrose (pH 7.4)] in a Dounce homogeniser.9 The fibril suspension was diluted into a solution of acid-extracted type I collagen (50 μg/ml) at 34 °C in a Na2HPO4 (62 mM)/KH2PO4 (15 mM) buffer, I 0.2, pH 7.4, which had been set up according to the “warm-start” procedure.10 The extraction of type I collagen from bovine skin and subsequent purification of a monomeric solution was as described previously.10 Importantly the telopeptides (extrahelical domains) of the collagen molecule, known to have a critical role in fibril assembly, were preserved intact. A droplet of the seed suspension was placed on a carbon-filmed 200-mesh copper grid and left to adsorb for 1 min, washed with ultrapure water and air-dried. Samples shown were unstained and imaged in a Tecnai-12 transmission electron microscope (FEI, Eindhoven, the Netherlands). (a) Typical fibril length fragment released from 13-day chick embryonic tendon. (b) Projection formed on blunt fibril ends after 30 min and (c and d) after 2 h incubation in collagen solution. (e) and (f) show long tapered projections after 24 h incubation. The arrows indicate the junction of the new tip projections with the seed fibril. (g) Plot of fraction of regrown fibril ends against time. The points are shown fitted to a function of the form f = b(1 − exp(− at)), indicating an average nucleation half-time of 2.1 h and a fraction (6%) of fibril ends that are unable to nucleate fresh growth. (h) Projection length distributions for two incubation times (2 and 24 h). The data are consistent with a near-uniform axial growth rate for the fibril projections. Scale bars, (a)–(d), 0.25 μm; (e) and (f), 2 μm.