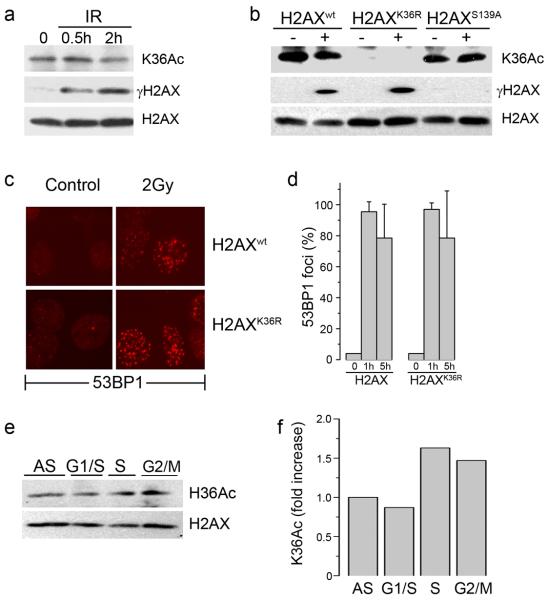
Figure 3. Acetylation of lysine 36 of H2AX is not altered by DNA damage.
(a) 293T cells expressing Flag-H2AX were irradiated (5 Gy) and chromatin associated histones isolated. H2AX, γH2AX and H2AXK36Ac were detected by western blot. (b) 293T cells expressing Flag-H2AX, Flag-H2AXK36R or Flag-H2AXS139A were irradiated (5Gy). H2AX, γH2AX and H2AXK36Ac were measured by western blot. (c) H2AX−/− MEFs expressing H2AXwt or H2AXK36R were irradiated (2Gy) and allowed to recover for 1hr. Cells were fixed and immunofluorescent staining carried out using anti-53BP1 antibody. (d) Quantitation of 53BP1 foci in cells expressing H2AX or H2AXK36R mutations. Cells were either untreated (0) or irradiated (2Gy) and the number of cells containing >5 foci counted 1h or 5h post-irradiation. (e) 293T cells stably expressing Flag-H2AX were either growing asynchronously (AS) or synchronized at the G1/S boundary, in S phase or G2/M as described in methods. H2AXK36Ac was monitored using AbK36Ac. (f) Western blots in (e) were scanned, and the ratio of K36 acetylation signal to total H2AX signal calculated.