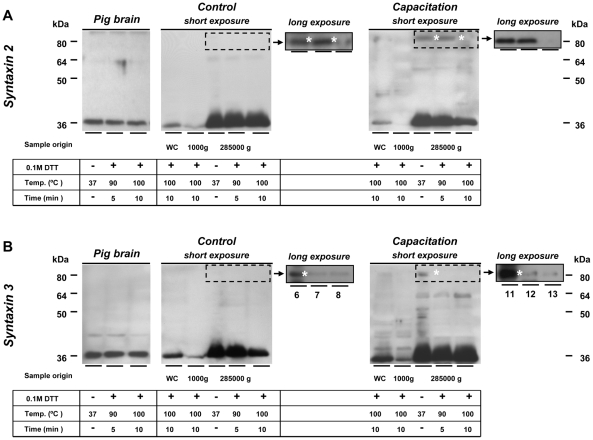
Figure 5. Syntaxin 2 and 3 interact with additional proteins and do so partly independent from capacitation.
(A): Syntaxin 2 was enriched in the cavitated membrane fraction (285000 g pellet) in the free 36 kDa form in both control and capacitated sperm cells. A weak signal at 80 kDa was observed in control cavitated membrane fractions (long exposure blot, marked with asterisks); this 80 kDa protein band was enhanced upon capacitation indicating a capacitation-enhanced but not capacitation-dependent interaction of syntaxin 2 with other proteins. (B): Syntaxin 3 also showed capacitation-independent formation of a 80 kDa SNARE-containing complex and this interaction (formation) is promoted by capacitation. However, due to the enrichment of both syntaxins in the cavitated membrane fractions and the relative small amount of complexed syntaxins (∼15%, see [20]) compared to the uncomplexed monomeric syntaxin, the dissociation of the syntaxins can not be observed clearly in these samples. 10 µg of total protein extract was used for all samples. WC = whole cell lysate; 1000 g = the remaining cavitated head fraction; 285000 g = cavitated membrane fraction.