Abstract
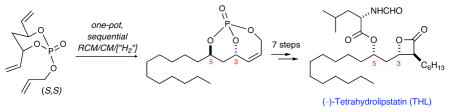
An efficient synthesis of (−)-tetrahydrolipstatin (THL) is reported. This method takes advantage of a phosphate tether-mediated, one-pot, sequential RCM/CM/hydrogenation protocol to deliver THL in 8 total steps from a readily prepared (S,S)-triene. The strategy incorporates selective cross metathesis, regio-selective hydrogenation, regio- and diastereoselective cuprate addition and Mitsunobu inversion for installation of the C5 formamide ester subunit.
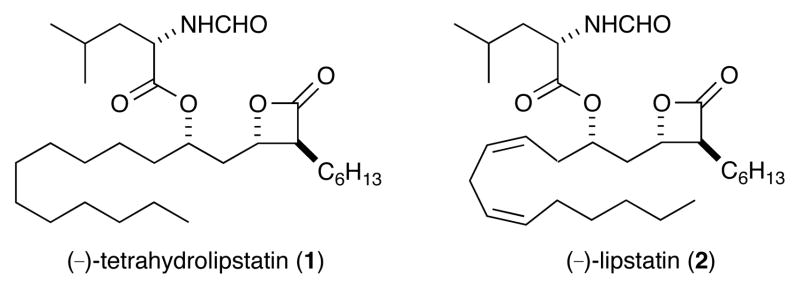
(−)-Tetrahydrolipstatin (THL, 1) is an anti-obesity drug marketed under generic name Orlistat® and is a stable saturated form of the naturally occuring lipstatin (2) (Figure 1). Lipstatin is a protein-reactive natural product and an irreversible pancreatic lipase inhibitor which was first isolated in 1987 from Streptomyces toxytricini.1 The biological activity inherent to this family of molecules is based on the reactivity of the β-lactone moiety which is readily acylated by the pancreatic lipase enzyme. This process ultimately inhibits the enzyme reactivity aimed at hydrolyzing triglycerides to produce free fatty acids which are then readily absorbed into the dietary system.1b,2
Figure 1.
(−)-Tetrahydrolipstatin and (−)-Lipstatin
Recently, the discovery of selective inhibition of thioesterase activity of fatty acid synthase (FAS) in cancer cells has elevated the potential of Orlistat® as an anticancer drug.3,4 The inhibition of FAS stops both endothelial cell proliferation and angiogenesis and ultimately delays tumor progression in a variety of cancer cells. This promising activity highlights the broad and interesting biological profile of Orlistat® and has prompted renewed synthetic efforts and corresponding biology of THL, lipstatin and analogs thereof.4,5 Herein we report a concise total synthesis of (−)-tetrahydrolipstatin via a strategy utilizing a phosphate-tether-mediated, one-pot, sequential RCM/CM/hydrogenation pathway of triene (S,S)-7.6 Overall, the reported synthetic route comprises 9 total steps from the readily prepared diene diol-(S,S)-8 and highlights the utility of phosphate tethered processes and one-pot, multi-step operations.
The first total synthesis of THL was achieved in 1987 by Schneider and coworkers utilizing Wittig olefination and an aldol condensation as key steps in a non-stereoselective process.7 Numerous total syntheses,8 formal syntheses9 and synthetic analogues have followed this initial report, with the majority of synthetic pathways comprised of 14–25 steps. The shortest routes to THL reported to-date range from 10–12 steps using an array of synthetic strategies, including, (i) a 12-step anti-aldol approach,8i (ii) a 12-step diastereoselective allylation and crotylation sequence utilizing allyl/crotyltrifluoroborates,8n (iii) a 10-step tandem Mukaiyama-aldol lactonization,8o and (iv) a 12-step Prins cyclization approach.8q Other noteworthy strategies include substrate-controlled stereoselective hydrogenation to install the C2-C3 stereocenters,7c Lewis acid-catalyzed [2+2] cycloaddition,8f anti-aldol approach,8h substrate-controlled [2+3] cycloaddition,8j and diastereoselective aldol reaction with an embedded iron chiral auxiliary.8t
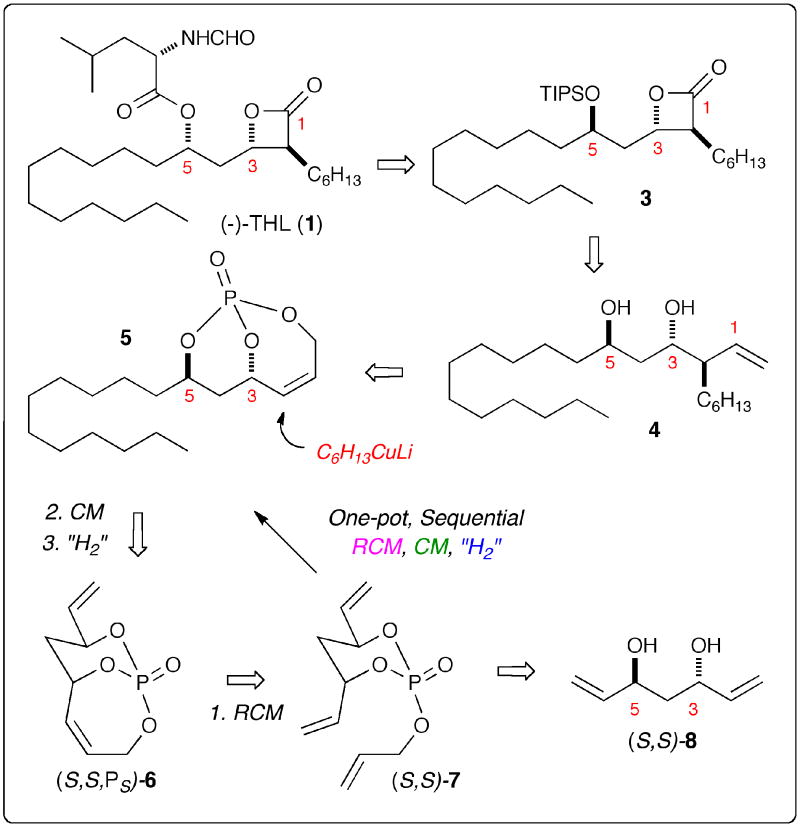
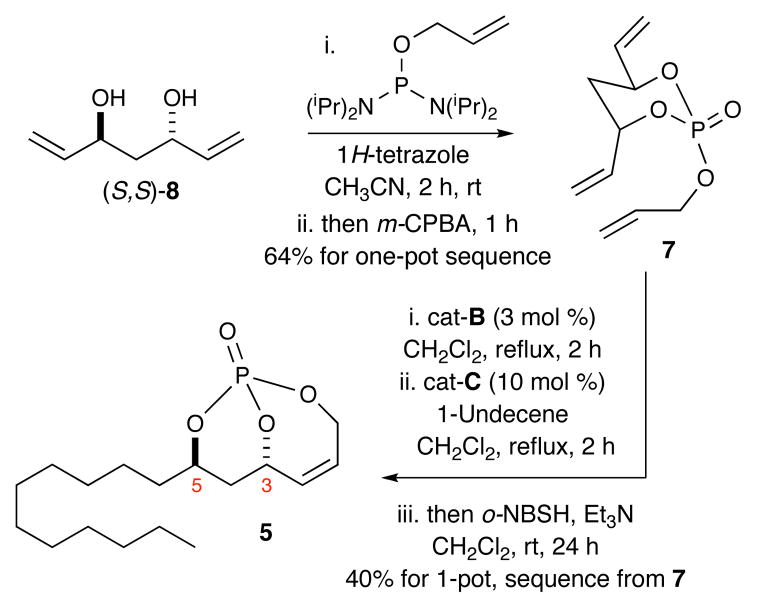
The route reported herein is highlighted in the retrosynthetic analysis shown in Scheme 1. THL (1) can be readily derived from β-lactone intermediate 3 via simple silyl deprotection and Mitsunobu esterification.7a β-Lactone 3 in turn can be synthesized from diol 4 via a 3-step sequence of TIPS-protection, ozonolysis/oxidation and lactonization. Diol 4 is obtained from 5 via diastereo-selective cuprate addition and phosphate tether removal under reductive conditions. Bicyclic phosphate 5 is in turn derived from (S,S,PS)-6 via cross-metathesis and regioselective hydrogenation. The bicyclic phosphate, (S,S,PS)-6,10 can be produced in a straightforward 2-step sequence from desymmetrization of the pseudo-C2-symmetric triene (S,S)-7 using an RCM/phosphate tether method inspired by Burke and coworkers.11 Triene (S,S)-7 is readily prepared from the C2-symmetric anti-diene diol (S,S)-812 in one step using phosphoramidite chemistry. Optimization was envisioned for a one-pot, sequential RCM/CM/hydrogenation sequence that would access 5 directly from triene (S,S)-7.
Scheme 1.
Retrosynthetic Analysis of (−)-Tetrahydrolipstatin
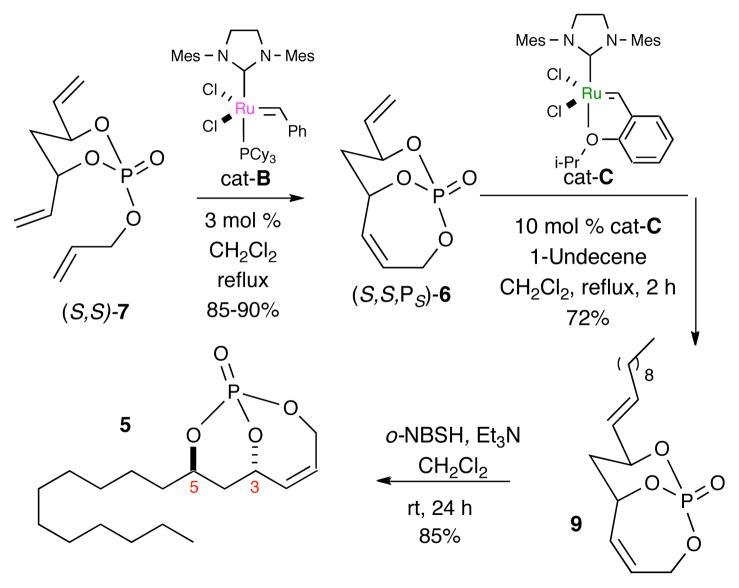
Initially, a linear approach was followed for the synthesis of 5 from bicyclic phosphate (S,S,PS)-6,13,14 which was synthesized via RCM desymmetrization of triene (S,S)-7 using [(IMesH2)(PCy3)(Cl)2Ru=CHPh; cat-B]15 (Scheme 2). Cross metathesis of phosphate (S,S,PS)-6 and 1-undecene, a type I olefin,16 using cat-C17 gave desired product 9 with > 99:1 E:Z selectivity. Regioselective hydrogenation of the exo-cyclic olefin under mild conditions (o-nitrobenzenesulfonyl hydrazine (o-NBSH), Et3N, CH2Cl2)18 via an in situ generated diimide afforded desired product 5 in 85% yield.10b
Scheme 2.
Stepwise RCM, CM and hydrogenation sequence
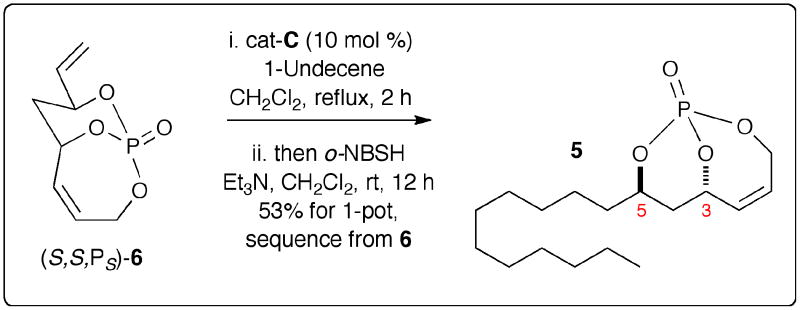
The development of a one-pot, RCM/CM/hydrogenation sequence was next investigated (Schemes 3 and 4). Recently a number of tandem and sequential protocols involving metathesis have followed the seminal report by Grubbs and coworkers.19 Initially, a one-pot, sequential CM/hydrogenation procedure was investigated using CH2Cl2 as a common solvent with no workup after the metathesis event. This CM/hydrogenation sequence proceeded smoothly yielding the desired hydrogenated product 5 in 53% yield with an average of 73% yield for each step (Scheme 3).
Scheme 3.
One-pot, Sequential CM/Hydrogenation Pathway
Scheme 4.
Phosphorodiamidite coupling and One-Pot, Sequential RCM/CM/Hydrogenation Sequence.
To further streamline the process, we optimized the previously reported two-step protocol for synthesizing triene 7 from the corresponding diene-diol 86a using a one-step process employing allyl tetraisopropylphosphoro-diamidite in the presence of tetrazole, followed by oxidation with m-CPBA. This method provided the desired triene 7 in 64% yield (Scheme 4).20 An RCM/CM/hydrogenation sequence from triene 7 was next investigated. Starting with triene (S,S)-7,5 RCM in the presence of cat-B and subsequent CM with cat-C, followed by hydrogenation with o-NBSH, gave the desired hydrogenated product 5 in 40% yield along with 7% of the hydrogenated product of unreacted (S,S,PS)-6 phosphate [5:1 ratio].21 Overall, this method represents an average yield of 74% for each step. Moreover, it simplifies the synthesis of 5 to a 2-step protocol from diene-diol (S,S)-8.
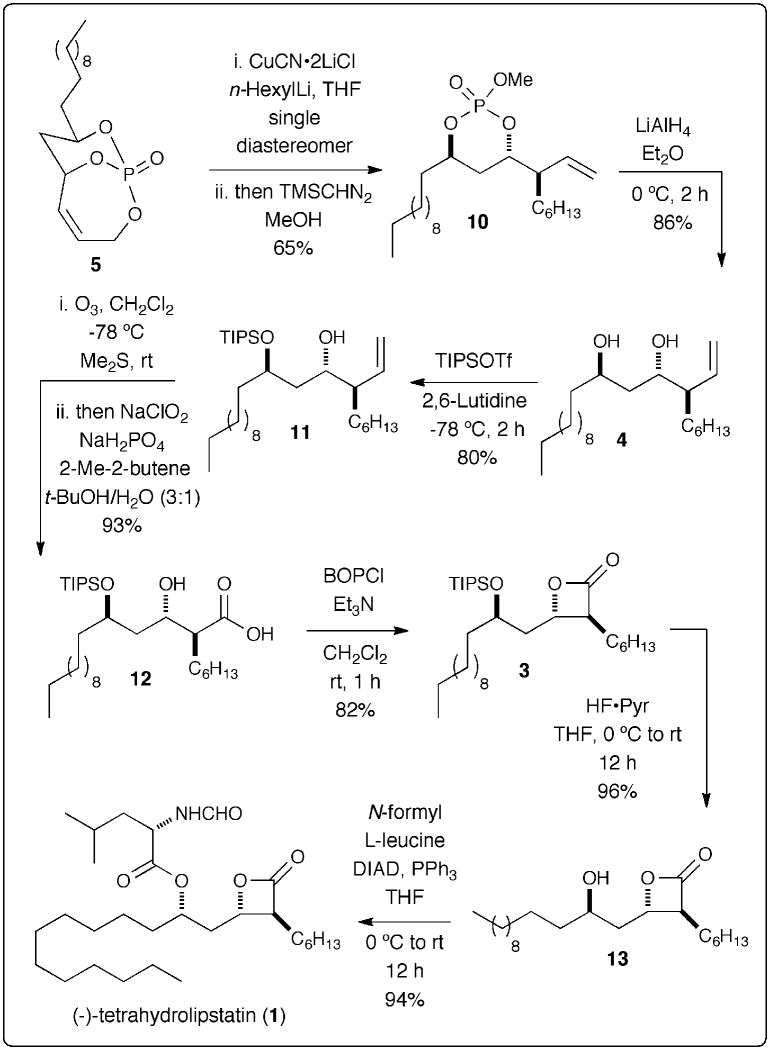
With 5 in place, 7-steps were required to complete the total synthesis of THL (1) (Scheme 5). SN2′ addition on endo-cyclic olefin with in situ generated organocuprate reagent followed by methylation with TMSCHN2 proceeds with high regio- and diastereoselectivity giving desired monocyclic phosphate 10.6 The phosphate tether was next removed under reductive conditions with the use of two equivalents of LiAlH4 affording diol 4 with all the desired stereocenters that are carried through until the last step of the sequence where inversion of the C5 stereocenter is carried out with Mitsunobu esterification. Selective silyl protection of the sterically more accessable C5 alcohol in 4 gave the desired silylether adduct 11 in 80% yield.22 Conversion to carboxylic acid 12 was accomplished via ozonolysis/Pinnick oxidation protocol affording 12 in an overall 93% yield. The TIPS-protected β-lactone 3 was next readily accessed via lactonization of β-hydroxy carboxylic acid 12 in the presence of BOPCl through a mixed anhydride intermediate. Ensuing TIPS-deprotection under mild basic conditions (HF·pyr) followed by esterification with N-formyl-L-leucine under Mitsunobu inversion conditions (DIAD, PPh3) developed by Schneider7a afforded the desired final product tetrahydrolipstatin (1) in 94% yield with all matching characterization of the reported data.7
Scheme 5.
Total Synthesis of (−)-Tetrahydrolipstatin (1)
In conclusion, a successful synthesis of (−)-tetrahydrolipstatin has been developed that incorporates a phosphate tether approach starting from diene-diol (S,S)-8. Overall, a 9-step route from diene-diol (S,S)-8 employing a phosphorodiamidite coupling and one-pot, sequential RCM/CM/hydrogenation sequence has been developed. Current efforts are focused on further optimization of the aforementioned one-pot, sequential RCM/CM/hydrogenation process, phosphorodiamidite coupling as well as additional phosphate tether approaches towards bioactive natural products containing 1,3-anti-diol subunits. The use of this one-pot, sequential RCM/CM/-hydrogenation sequence towards the synthesis of other bioactive natural products is ongoing and will be reported in due course.
Supplementary Material
Acknowledgments
This investigation was generously supported by funds provided by the National Institute of General Medical Sciences (NIH RO1 GM077309). The authors thank Dr. Justin Douglas and Sarah Neuenswander for assistance with NMR measurements and Dr. Todd Williams for HRMS analysis. The authors also thank Materia, Inc. for supplying metathesis catalyst and helpful suggestions.
Footnotes
Supporting Information Available Experimental details and spectroscopic data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Weibel EK, Hadvary P, Hochuli E, Kupfer E, Lengsfeld H. J Antibiot. 1987;40:1081–1085. doi: 10.7164/antibiotics.40.1081. [DOI] [PubMed] [Google Scholar]; (b) Hochuli E, Kupfer R, Maurer R, Meister W, Mercadal Y, Schmidt K. J Antibiot. 1987;40:1086–1091. doi: 10.7164/antibiotics.40.1086. [DOI] [PubMed] [Google Scholar]
- 2.(a) Stalder H, Schneider PR, Oesterhelt G. Helv Chim Acta. 1990;73:1022–1036. [Google Scholar]; (b) Stalder H, Oesterhelt G. Helv Chim Acta. 1992;75:1593–1603. [Google Scholar]; (c) Drahl C, Cravatt BF, Sorensen E. Angew Chem, Int Ed Engl. 2005;44:5788–5809. doi: 10.1002/anie.200500900. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]; (b) Pemble CW, Johnson LC, Kridel SJ, Lowther WT. Nat Struct Mol Biol. 2007;14:704–709. doi: 10.1038/nsmb1265. [DOI] [PubMed] [Google Scholar]; (c) Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Cancer Res. 2007;67:1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 4.Yang P-Y, Liu K, Ngai MH, Lear MJ, Wenk MR, Yao SQ. J Am Chem Soc. 2010;132:656–666. doi: 10.1021/ja907716f. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 5.(a) Richardson RD, Yatsandra OG, Zancanella M, Knowles LM, Cieplak P, Romo D, Jeffrey W, Smith JW. J Med Chem. 2008;51:5285–5296. doi: 10.1021/jm800321h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ortar G, Bisogno T, Ligresti A, Morera E, Nalli M, Di Marzo V. J Med Chem. 2008;51:6970–6979. doi: 10.1021/jm800978m. [DOI] [PubMed] [Google Scholar]
- 6.(a) Whitehead A, McReynolds MD, Moore JD, Hanson PR. Org Lett. 2005;7:3375–3378. doi: 10.1021/ol0512886. [DOI] [PubMed] [Google Scholar]; (b) Thomas CD, McParland JM, Hanson PR. Eur J Org Chem. 2009:5487–5500. doi: 10.1002/ejoc.200900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbier P, Schneider F. Helv Chim Acta. 1987;70:196–202.Barbier P, Schneider F, Widmer U. Helv Chim Acta. 1987;70:1412–1418.Barbier P, Schneider F. J Org Chem. 1988;53:1218–1221.(d) For the first synthetic route to (−)-lipstatin, see: Pons JM, Pommier A, Lerpiniere J, Kocienski P. J Chem Soc Perkin Trans 1. 1993:1549–1551.
- 8.(a) Pons JM, Kocienski P. Tetrahedron Lett. 1989;30:1833–1836. [Google Scholar]; (b) Fleming I, Lawrence NJ. Tetrahedron Lett. 1990;31:3645–3648. [Google Scholar]; (c) Case-Green SC, Davies SG, Hedgecock CJR. Synlett. 1991:781–782. [Google Scholar]; (d) Chadha NK, Batcho AD, Tang PC, Courtney LF, Cook CM, Wovkkulich PM, Uskokovic MR. J Org Chem. 1991;56:4714–4718. [Google Scholar]; (e) Hanessian S, Tehim A, Chen P. J Org Chem. 1993;58:7768–7781. [Google Scholar]; (f) Pommier A, Pons JM. Synthesis. 1994:1294–1300. [Google Scholar]; (g) Fleming I, Lawrence NJ. J Chem Soc Perkin Trans 1. 1998:2679–2686. [Google Scholar]; (h) Ghosh A, Liu C. Chem Commun. 1999:1743–1744. doi: 10.1039/A904533C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Paterson I, Doughty VA. Tetrahedron Lett. 1999;40:393–394. [Google Scholar]; (j) Dirat O, Kouklovsky C, Langlois Y. Org Lett. 1999;1:753–755. doi: 10.1021/ol990734k. [DOI] [PubMed] [Google Scholar]; (k) Ghosh A, Fidanze S. Org Lett. 2000;2:2405–2407. doi: 10.1021/ol000070a. [DOI] [PubMed] [Google Scholar]; (l) Sato M, Nakashima H, Hanada K, Hayashi M, Honzumi M, Taniguchi T, Ogasawara K. Tetrahedron Lett. 2001;42:2833–2837. [Google Scholar]; (m) Bodkin JA, Humphries EJ, McLeod MD. Tetrahedron Lett. 2003;44:2869–2872. [Google Scholar]; (n) Thadani AN, Batey RA. Tetrahedron Lett. 2003;44:8051–8055. [Google Scholar]; (o) Yin J, Yang XB, Chen ZX, Zhang YH. Chin Chem Lett. 2005;16:1448–1450. [Google Scholar]; (p) Yadav JS, Vishweshwar Rao K, Prasad AR. Synthesis. 2006:3888–3894. [Google Scholar]; (q) Yadav JS, Sridhar Reddy M, Prasad AR. Tetrahedron Lett. 2006;47:4995–4998. [Google Scholar]; (r) Yadav JS, Vishweshwar Rao K, Sridhar Reddy M, Prasad AR. Tetrahedron Lett. 2006;47:4393–4395. [Google Scholar]; (s) Kumaraswamy G, Markondaiah B. Tetrahedron Lett. 2008;49:327–330. [Google Scholar]; (t) Case-Green SC, Davies SG, Roberts PM, Russell AJ, Thomson JE. Tetrahedron:Asymmetry. 2008;19:2620–2631. [Google Scholar]; (u) Raghavan S, Rathore K. Synlett. 2009:1285–1288. [Google Scholar]; (v) Ghosh A, Shurrush K, Kulkarni S. J Org Chem. 2009;74:4508–4518. doi: 10.1021/jo900642f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (w) Raghavan S, Rathore K. Tetrahedron. 2009;65:10083–10092. [Google Scholar]
- 9.(a) Landi JL, Garofalo LM, Jr, Ramig K. Tetrahedron Lett. 1993;34:277–280. [Google Scholar]; (b) Wedler C, Costisella B, Schick H. J Org Chem. 1999;64:5301–5303. doi: 10.1021/jo980292a. [DOI] [PubMed] [Google Scholar]; (c) Sharma A, Chattopadhyay S. J Org Chem. 1999;64:8059–8062. doi: 10.1021/jo990370+. [DOI] [PubMed] [Google Scholar]; (d) Polkowska J, Lukaszewicz E, Kiegiel J, Jurczak J. Tetrahedron Lett. 2004;45:3873–3875. [Google Scholar]
- 10.(a) Waetzig JD, Hanson PR. Org Lett. 2006;8:1673–1676. doi: 10.1021/ol0602809. [DOI] [PubMed] [Google Scholar]; (b) Waetzig JD, Hanson PR. Org Lett. 2008;10:109–112. doi: 10.1021/ol7025944. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Whitehead A, Waetzig JD, Thomas CD, Hanson PR. Org Lett. 2008;10:1421–1424. doi: 10.1021/ol8001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Burke SD, Muller N, Beaudry CM. Org Lett. 1999;1:1827–1829. doi: 10.1021/ol9910971. [DOI] [PubMed] [Google Scholar]; (b) Burke SD, Voight EA. Org Lett. 2000;3:237–240. doi: 10.1021/ol006887l. [DOI] [PubMed] [Google Scholar]
- 12.(a) Diene diol (S,S)-8 can be synthesized from the corresponding 1,5-dichloropentane-2,4-diol (Rychnovsky diol) in one step (see reference 6). For synthesis of the Rychnovsky diol, see: Rychnovsky SD, Griesgraber G, Powers JP. Org Synth. 2000;77:1–11.
- 13.The enantiomeric pair of bicyclic phosphates, (R,R,PR)-6 and (S,S,PS)-6, have been previously utilized for generation of the C1–C14 and C15–C30 segments of dolabelide C and gram scale syntheses are now routinely carried out in our laboratory, see reference 10.
-
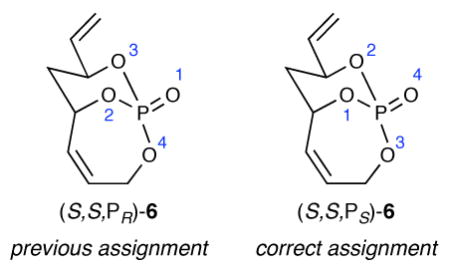
14.We previously assigned the stereochemical descriptor at phosphorus in (S,S,PS)-6 as PR (see reference 6b). Workers at Chemical Abstracts Service kindly noted that Cahn-Ingold-Prelog priority rules dictate that “Contributions by d-orbitals to bonds of quadriligant atoms are neglected”, and hence the P=O in 6 should be treated as a P–O with assignment of least priority, see page 391 in Cahn RS, Ingold C, Prelog V. Angew Chem Int Ed. 1966;5:385–415.
- 15.Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q.Use of Grubbs first generation catalyst (PCy3)2(Cl)2Ru=CHPh (cat-A)[see reference 10] gave poorer yields [see reference 6a].
- 16.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360–1137. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 17.Kingsbury JS, Harrity JPA, Bonitatebus PJ, Jr, Hoveyda AH. J Am Chem Soc. 1999;121:791–799. [Google Scholar]
- 18.(a) Myers AG, Zheng B, Movassaghi M. J Org Chem. 1997;62:7507. doi: 10.1021/jo9710137. [DOI] [PubMed] [Google Scholar]; (b) O’Doherty GA, Haukaas MH. Org Lett. 2002;4:1771–1774. doi: 10.1021/ol025844x. [DOI] [PubMed] [Google Scholar]; (c) Buszek KR, Brown N. J Org Chem. 2007;72:3125–3128. doi: 10.1021/jo0622173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For Tandem metathesis/hydrogenation, see: Louie J, Bielawski CW, Grubbs RH. J Am Chem Soc. 2001;123:11312–11313. doi: 10.1021/ja016431e.For CM/Wittig Olefination, see: Murelli RP, Snapper ML. Org Lett. 2007;9:1749–1752. doi: 10.1021/ol070445t.For RCM/Oxidation Scholte AA, An M-H, Snapper ML. Org Lett. 2006;8:4759–4762. doi: 10.1021/ol061837n.Seigal BA, Fajardo C, Snapper ML. J Am Chem Soc. 2005;127:16329–16332. doi: 10.1021/ja055806j.For tandem RCM/CM and Hydrogenation Quinn KL, Curto JM, McGrath KP, Biddick NA. Tetrahedron Lett. 2009;50:7121–7123.Quinn KJ, Isaacs AK, Arvary RA. Org Lett. 2004;6:4143–4145. doi: 10.1021/ol040047f.Virolleaud M-A, Bressy C, Piva O. Tetrahedron Lett. 2003;44:8081–8084.Virolleaud M-A, Piva O. Tetrahedron Lett. 2007;48:1417–1420.For CM/Hydrogenation/Cyclization Cossy J, Bargiggia F, BouzBouz S. Org Lett. 2003;5:459–462. doi: 10.1021/ol027347m.For tandem CM/amidation, see: Ferrie L, Bouzbouz S, Cossy J. Org Lett. 2009;11:5446–5448. doi: 10.1021/ol9021386.
- 20.Bannwarth W, Trzeciak A. Helv Chim Acta. 1987;70:175–186. [Google Scholar]
- 21.Use of the Hoveyda-Grubbs 2nd generation catalyst (cat-C) in the initial RCM gave lower yields during the RCM/CM/[H2] sequence compared to sequential use of cat-B for the RCM and cat-C for the CM.
- 22.For selective silylation of similar 1,3-diols, see: Soltani O, De Brabander JK. Org Lett. 2005;7:2791–2793. doi: 10.1021/ol0510769.Yamaguchi M, Hirao I. Tetrahedron Lett. 1983;24:391–394.See also: Morris J, Wishka DG. Tetrahedron Lett. 1986;27:803–806.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.