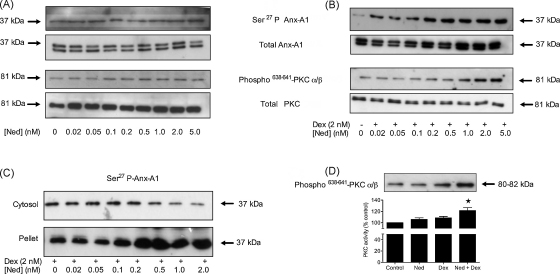
Fig. 2.
Nedocromil potentiates the effect of dexamethasone on Anx-A1 and PKCα/β phosphorylation and protein translocation to the plasma membrane fraction. (Panel A) Nedocromil itself, over a range of concentrations, has a negligible effect on the concentration of Ser27 phospho Anx-A1 in U937 cell lysates or PKCα/β phosphorylation when compared to untreated samples. Total Anx-A1 (sometimes shown as a 33/37 kDa doublet) and PKC is shown for reference purposes. (Panel B) In the presence of 2 nM dexamethasone, escalating concentrations (0.02–5.0 nM) of nedocromil potentiate (by a further 3–4-fold) the phosphorylation of both Anx-A1 and PKCα/β. Total Anx-A1 (sometimes shown as a 33/37 kDa doublet) and PKC is shown for reference purposes. (Panel C) In the presence of a fixed concentration of dexamethasone (2 nM), nedocromil (0.02–2.0 nM) potentiates the translocation of Ser27 phospho Anx-A1 from the cytosol into the 13,000 × g particulate fraction of U937 cells. (Panel D) The membrane accumulation of activated PKCα/β is promoted (>2-fold) by the combination of nedocromil (0.5 nM) and dexamethasone (2 nM; see blot insert) and this is reflected in an increase in enzyme activity as measured in the 100,000 × g membrane fraction. *P < 0.05 relative to nedocromil of dexamethasone alone. All experiments were done at least three times and the blots are representatives from one of these experiments. Densitometry was performed as described in the methods and the optical density units normalised by comparison to α-tubulin. Data are expressed as mean ± S.E.M. Statistical differences between groups were established using one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test.