Figure 3.
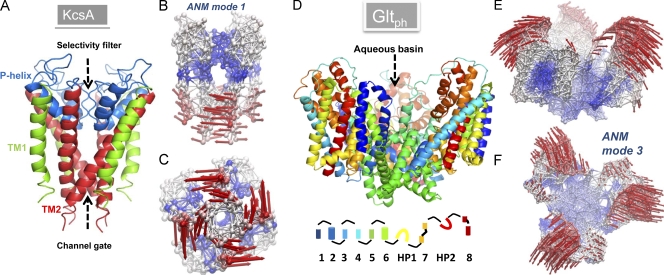
Structure and softest modes for the potassium channel KcsA (A–C) and glutamate transporter GltPh (D–F). (A and D) The respective ribbon diagrams of KcsA (PDB accession no. 1K4C) and GltPh (PDB accession no. 1XFH) structures. KcsA is a homotetramer. The outer (TM1) and inner (TM2) helices of each monomer span the bilayer, while the short P helix spans half the bilayer, enclosing the selectivity filter. GltPh is a homotrimer. The diagram at the bottom represents the color code for the eight TM helices, and the two reentrant loops, HP1 and HP2. (B and C) The ANM representation of KcsA, colored-coded by the mobility of residues in ANM mode 1. Blue, almost rigid; red, most mobile. The arrows indicate the direction of motion in this mode. The lower portions of the helices undergo the largest motions, inducing an enlargement in the channel gate lined by the four TM2 helices. (E and F) The ANM results for GltPh in the outward-facing state, colored according to mobility in ANM mode 3 (softest nondegenerate mode). The extracellular regions of the three subunits surrounding the central aqueous basin undergo concerted opening/closing movements to alternatively occlude/expose the central cavity to solvent and solutes.