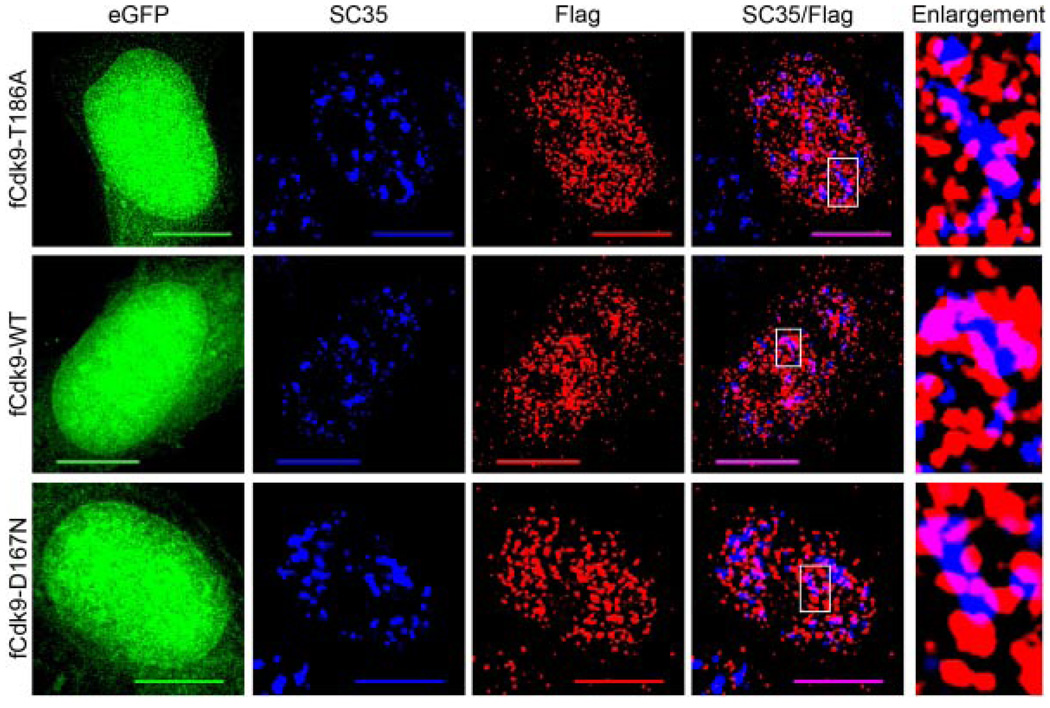
Fig. 4.
Cdk9 does not require Thr186 phosphorylation or kinaseactivity for nuclear speckle localization. HeLa cells grown on glass coverslips were transfected with 500 ng of either: pBABE-Flag-Cdk9T186A-IRES-eGFP (fCdk9-T186A, upper parts), pBABE-Flag-Cdk9-IRES-eGFP (fCdk9-WT, middle parts), or pBABE-Flag-Cdk9D167N-IRES-eGFP (fCdk9-D167N, bottom parts) and fixed at 24 h post-transfection. The fixed cells were then immunolabeled for Flag (red) and SC35 (blue). The eGFP (green) was used as an internal control in conjunction with the Flag signal for assessing cells with similar expression levels. Immunoblots were also conducted to confirm that the expression levels of eGFP and the epitope-tagged Cdk9 proteins were similar in all cultures examined (data not shown). As can be seen, all three of the Flag-tagged Cdk9 proteins co-localize with SC35 (purple) to a similar extent, and show a similar non-nucleolar distribution throughout the nucleoplasm. An enlargement of a nuclear speckle from each of the three cells (boxed region on SC35/Flag parts) is shown on the right. Bar, 10 µm.