Table 4.
Effects of the Micheal acceptor on the intramolecular Stetter reaction. 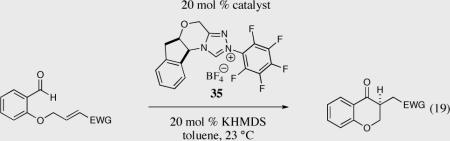
| Entry | Substrate | Product | Yield % | ee% |
|---|---|---|---|---|
| 1 |
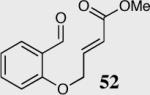
|
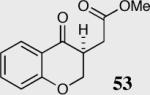
|
94 | 95 |
| 2b |
|
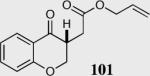
|
94 | 93 |
| 3b |
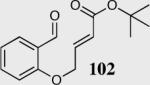
|
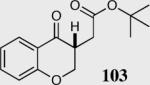
|
94 | 97 |
| 4 |
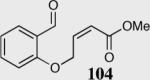
|
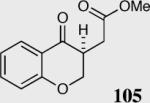
|
80 | 22 |
| 5b |
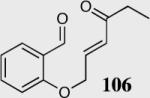
|
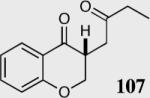
|
94 | 92 |
| 6 |
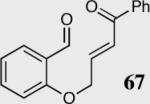
|
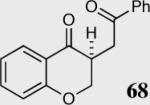
|
94 | 78 |
| 7 |
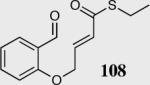
|
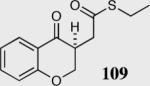
|
85 | 70 |
| 8b |
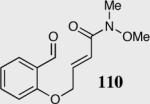
|
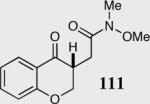
|
94 | 92 |
| 9 |
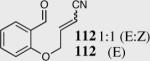
|
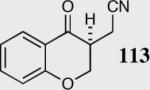
|
88 | 80 |
| 10* | 80 | 78 | ||
| 11b |
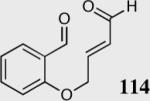
|
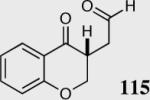
|
50 | 30 |
20 mol % of catalsyt 33 was used.
Opposite antipode of the chiral triazolium salt 35 was used.
