Table 8.
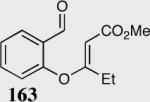
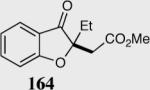
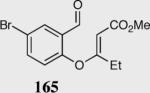
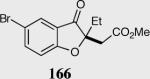
Formation of quaternary stereocenters from aromatic aldehydes via the asymmetric Stetter reaction. 
| Entry | Substrate | Product | Yield % | ee% |
|---|---|---|---|---|
| 1 |
|
|
96 | 97 |
| 2 |
|
|
92 | 89 |
| 3 |
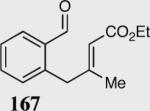
|
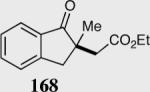
|
95 | 99 |
| 4 |
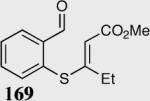
|
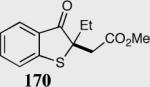
|
95 | 92 |
| 95a | 92a | |||
| 5 |
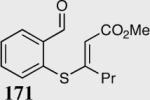
|
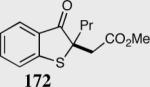
|
54 | 87 |
| 83a | 98a | |||
| 6 |
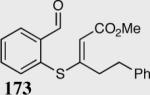
|
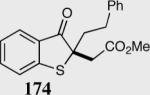
|
33 | 88 |
| 91a | 99a | |||
| 7 |
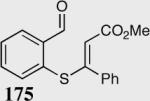
|
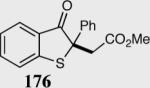
|
11 | 82 |
| 15a | 82a | |||
| 8b |
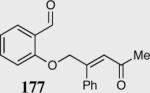
|
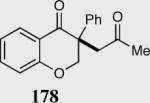
|
55 | 99 |
| 9 |
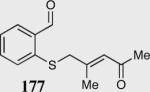
|
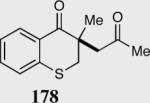
|
0 | --- |
| 11c | >99c |
KOt-Bu was used as the base.
Catalyst added in two portions.
Catalyst 40 was used.
