Abstract
New hybrid bis(phosphine)(pyrazole)borate tripodal ligands ([PhBPtBu2(pz’)]−) are reported that support pseudotetrahedral iron in the oxidation states +1, +2, +3, and +4. The higher oxidation states are stabilized by a terminal Fe≡NR linkage. Of particular interest is the generation and thorough characterization of an S = 1 FeIV≡NR+ imide cation using this new ligand system. The latter species can be observed electrochemically and spectroscopically, and its solid-state crystal structure is reported.
Although implicated as intermediates in group transfer reactions,1 mid-to-late first row transition metals (e.g., Mn, Fe, Co, Ni) featuring terminal imido/nitrene functionalities are rare.2 To date the only examples of structurally characterized mononuclear iron imides are those supported by tris(phosphino)borate ligands.3 These species have been reported in the Fe(III) and Fe(II) oxidation states. In the Fe(III) state, they have been accessed via oxidative nitrene transfer from organic azides using low valent FeI precursors. The [PhBPR 3]FeIII(NR) complexes that have been isolated all show electrochemically reversible FeIII/II couples, and chemical reduction typically provides their corresponding d6 {[PhBPR 3]FeII(NR)}− analogues in high yield. Well-defined FeIV=NR species have proven generally more elusive,4 though thoroughly characterized examples of FeIV=O species are now well known.5 To the best of our knowledge the single report of a complex that can be formulated as an Fe(IV) imide concerns Lee’s tetranuclear cluster Fe4(µ3-NtBu)4(NtBu)Cl3, isolated in only 1–2% yield.6 Mössbauer data for this species were consistent with a cluster featuring three Fe(III) centers and one Fe(IV) center, the latter most likely indicative of the FeIV=NtBu terminal linkage. Herein we describe a new hybrid pyrazolyl/phosphino-borate ligand that can support pseudotetrahedral iron in the +1, +2, +3, and +4 oxidation states. The +3 and +4 oxidation states are stabilized by the terminal Fe≡NR imide linkage.
Access to the required bis(phosphino)pyrazolylborate7 ligand is achieved by initial preparation of the bis(phosphino)borane precursor PhB(CH2PtBu2)2 (1) via metathesis between PhBCl2 and two equivalents of LiCH2PtBu2 (Scheme 1). Reaction of [pz]Li with 1 (pz = pyrazolyl), followed immediately by salt metathesis with TlPF6, leads to the clean formation of solid white [PhBPtBu 2(pz)]Tl (2a) in 66% isolated yield.8 The use of the bulky LiCH2PtBu2 carbanion is critically important in the preparation of this type of hybrid borate ligand because (i) effective di- rather than tri-substitution at boron can be achieved, which could not be realized using less hindered carbanions such as LiCH2PiPr2 and LiCH2PPh2; (ii) the borane product, PhB(CH2PtBu2)2 (1), does not appear to dimerize to an appreciable degree in solution. This fact allows the efficient introduction of a third donor arm.
Scheme 1.
Metathesis of 2a with FeCl2 leads to the clean formation of the high spin (S = 2) complex [PhBPtBu2(pz)]FeCl (3a), isolated in 69% yield as a yellow crystalline solid. Solution 1H NMR data for 3a are suggestive of a monomeric structure in solution, in accord with the calculated solution Evans’ method magnetic moment at 295 K (5.2 µB).9 However, the 100 K X-ray crystal structure of 3a establishes a dimeric structure with bridging chlorides and a crystallographic center of symmetry in the solid-state (see SI for details).
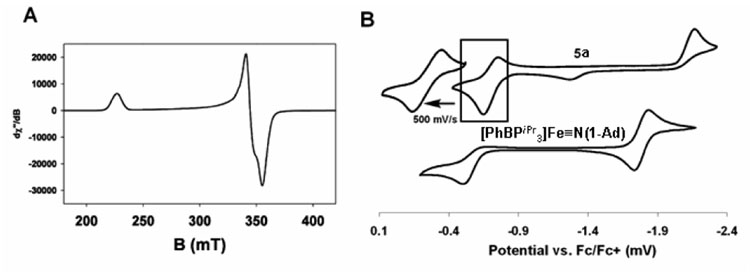
Following an overall methodology that proved effective for the synthesis of Fe(III) imides previously, one-electron reduction of 3a with sodium/mercury amalgam in the presence of excess PMe3 generates the high spin d7 precursor [PhBPtBu 2(pz)]Fe(PMe3) (4a) as a pale green crystalline solid (65% isolated yield; µeff = 4.2 µB). The reaction between 4a and two equivalents of 1-adamantylazide (1-AdN3) generates the expected Fe(III) imide [PhBPtBu 2(pz)]Fe≡NAd (5a) as a red-brown low spin species (2.0 µB in C6D6), in addition to a stoichiometric equivalent of (1-Ad)N=PMe3. The EPR spectrum of 5a displays a rhombic signal that was simulated (see SI) to provide the g values g1 = 2.96, g2 = 1.95, and g3 = 1.88 (Figure 1A), a spectrum similar to those of related [PhBPR3]FeIII≡NR imides.
Figure 1.
(A) EPR spectrum of 5a with g1 = 2.96, g2 = 1.95, and g3 = 1.88 (in 2-methyltetrahydrofuran glass at 20 K, 9.474 GHz). (B) Cyclic voltammetry of 5a (0.40 M [nBuN4][ClO4] in THF, scan rate = 100 mV/s (full), 500 mV/s (inset)), and [PhBPiPr 3]Fe≡NAd (0.40 M [nBuN4][PF6] in THF).
The cyclic voltammetry of 5a reveals very different features from those observed for [PhBPR3]FeIII(NR) imides (Figure 1B). For example, the cyclic voltammagram of previously reported [PhBPiPr 3]Fe≡NAd3b features a fully reversible reductive wave at −1.79 V and an irreversible oxidative wave at ca. −0.45 V. This latter process presumably reflects a one-electron oxidation to an unstable Fe(IV) species. By contrast, complex 5a exhibits a completely irreversible reductive wave at −2.20 V, indicating that the Fe(II) imide anion is, in this case, unstable. The oxidative irreversible wave at −1.26 V appears only after scanning through the −2.20 V wave, indicating that it represents a byproduct of the one-electron reduction of 5a. More interesting, however, is the presence of a quasi-reversible feature at −0.72 V for 5a (at 100 mV/s; 22 °C). This wave becomes fully reversible at ambient temperature when the scan rate is increased to 500 mV/s. It represents an FeIV/III redox couple and suggests that “{[PhBPtBu 2(pz)]FeIV≡NAd}+” might be modestly stable.
In accord with these electrochemical data, 5a can be chemically oxidized with [Fc][B(ArF)4] (ArF = 3,5-(CF3)2-C6H3) at low temperature (−50 °C) in THF solution to generate a green, cationic species formulated as {[PhBPtBu 2(pz)]FeIV≡NAd}{B(ArF)4} (6a). A single set of paramagnetic resonances is observed for 6a in its 1H NMR spectrum at −50 °C (see SI), distinct from the resonances observed for 5a. Using an optical dip-probe assembly, the appearance of absorption bands at 580 nm and 677 nm are readily observed at low temperature upon addition of the ferrocenium oxidant (see SI). We also established that the addition of 1 equiv of CoCp2 to 6a generated in situ in THF-d8 solution regenerated 5a cleanly. The half-life of 6a is approximately 50 min at −40 °C in THF-d8 and it must therefore be manipulated at temperatures below −40 °C. Solution magnetic data collected at low temperature (µeff = 3.1(2) µB in THF-d8, 222 K; avg of 4 runs) indicate two unpaired electrons (S = 1), consistent with the ground state electronic configuration (dz2)2(dxy)1(dx2–y2)1(dxz)0(dyz)0.
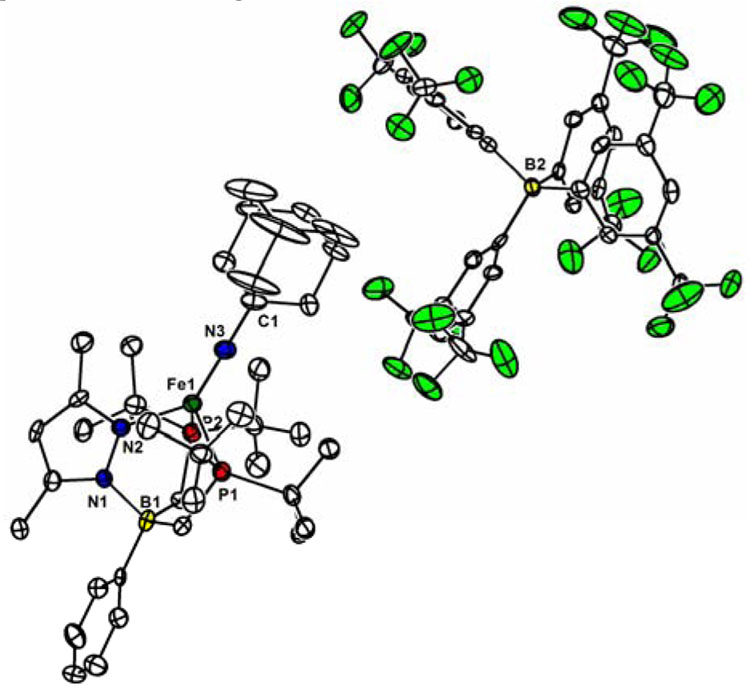
We undertook a crystallographic investigation of 5a and 6a to confirm their connectivities and to examine their Fe-N bond distances and Fe-Nimide-C bond angles for comparison with [PhBPR 3]Fe≡NR imides. For the case of 5a, the crystals that we obtained were twinned, regardless of the method employed for crystallization. Its structure could nonetheless be refined isotropically to confirm its pseudotetrahedral geometry (see SI). Evident from its isotropic structure is an expectedly short Fe-Nimide bond distance (1.63 Å) and a nearly linear C-N3-Fe angle (169°).3
Owing to the thermal instability of 6a, its XRD analysis proved to be a more challenging experiment. Single green crystals could be obtained by storing a THF/petroleum ether solution at −78 °C for several days, and XRD analysis confirmed its proposed assignment (see SI). To obtain a better quality data set we set out to prepare an analogue of 6a of greater kinetic stability. We thus pursued a [PhBPtBu 2(pz’)]− derivative substituted by methyl groups at the 3 and 5 positions. The required precursor ([PhBPtBu 2(pzMe2)]Tl, 2b) and an analogous series of iron complexes (3b – 6b, Scheme 1) were readily prepared by the same procedures described above. The imide cation 6b indeed exhibits far greater thermal stability than 6a. It’s cyclic voltammetry is very similar to that of 6a, and the FeIV/III redox couple is reversible even at slower scan rates (e.g., 100 mV/s). 6b can even be isolated in pure form at ambient temperature (86.7% yield) and manipulated without appreciable degredation for short periods. X-ray data sets were obtained for 5b (disordered structure; see SI) and 6b. The anisotropically refined X-ray crystal structure of {[PhBPtBu 2(pzMe2)]FeIV≡NAd}{B(ArF)4} 6b is shown in Figure 2. The structure reveals the anticipated pseudotehedral iron cation and its tetra(aryl)borate counter-anion. The Fe-N3 bond distance (1.634(4) Å) and the Fe-Nimide-C bond angle (176.2(3)°) are similar to the parameters obtained for crystallographically characterized [PhBP3]Fe imides in the +3 and +2 oxidation states. Assuming that the two unpaired electrons of 6b reside in relatively nonbonding d-orbitals, as predicted from simple MO considerations,2b,3c the Fe-N bond should retain its triple bond character (i.e., FeIV≡NR+) and the Fe-N distance is therefore not expected to change to a large extent upon oxidation.
Figure 2.
Anisotropically refined thermal ellipsoid representation of {[PhBPtBu 2(pzMe2)]FeIV≡NAd}{B(ArF)4} (6b); two molecules of THF and all hydrogen atoms have been omitted for clarity; Fe-N3: 1.634(4) Å, Fe-N3-C1: 176.2(3)°.
To conclude, we have introduced the hybrid phosphine/pyrazole borate ligands [PhBPtBu 2(pz’)]−, and begun to explore their utility in the context of nitrene group transfer to iron. Whereas iron imides had been previously obtained in the +3 and +2 oxidation states using [PhBPR 3]Fe systems, we now find that imides in the +3 and +4 oxidation states are accessible using [PhBPtBu 2(pz’)]Fe systems. It is remarkable that terminally bonded L3Fe≡NR species have now been characterized in three distinct oxidation states using phosphine-borate ligands given the paucity of such species more generally. The cause of the increased stability of the FeIV≡NR linkage in the [PhBPtBu 2(pz’)]Fe system described herein is an interesting issue and might in part be attributed to (i) a cathodic shift in the FeIV/III potential by comparison to previous [PhBPR 3]Fe imide systems, which in turn might lend added stability to the ligand borate unit; (ii) and/or to the lower symmetry of the [PhBPtBu 2(pz’)] ligand, and the compatibility of this lower symmetry with a d4 triplet electronic configuration.
Supplementary Material
Detailed experimental procedures for 1, 2a,b – 6a,b, all characterization data, and crystallographic details for 3a, 5a, 5b, 6a, and 6b. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgement
We acknowledge Larry Henling for crystallographic assistance and Dr. Mark Mehn for assistance with EPR spectroscopy. We thank the NIH for financial support (GM 070757), and NPM is grateful for an NSF graduate fellowship.
References
- 1.(a) Li Z, Conser KR, Jacobsen EN. J. Am. Chem. Soc. 1993;115:5326. [Google Scholar]; (b) Evans DA, Faul MM, Bilodeau MT. J. Am. Chem. Soc. 1994;116:2742. [Google Scholar]; (c) DuBois J, Tomooka CS, Hong J, Carreira EM. Acc. Chem. Res. 1997;30:364. [Google Scholar]; (d) Wigley DE. Progress in Inorganic Chemistry. 1994;Vol. 42:239–482. [Google Scholar]; (e) Eikey RA, Abu-Omar MM. Coord. Chem. Rev. 2003;243:83. [Google Scholar]
- 2.For recent examples see: Mindiola DJ, Hillhouse GL. J. Am. Chem. Soc. 2001;123:4623. doi: 10.1021/ja010358a. Jenkins DM, Betley TA, Peters JC. J. Am. Chem. Soc. 2002;124:5272. doi: 10.1021/ja026852b. Eikey RA, Khan SI, Abu-Omar MM. Angew. Chem. Int. Ed. 2002;41:3592. doi: 10.1002/1521-3773(20021004)41:19<3591::AID-ANIE3591>3.0.CO;2-Z. Dai X, Kapoor P, Warren TH. J. Am. Chem. Soc. 2004;126:4798. doi: 10.1021/ja036308i. Hu X, Meyer K. J. Am. Chem. Soc. 2004;126:16322. doi: 10.1021/ja044271b.
- 3.(a) Brown SD, Betley TA, Peters JC. J. Am. Chem. Soc. 2003;125:322. doi: 10.1021/ja028448i. [DOI] [PubMed] [Google Scholar]; (b) Betley TA, Peters JC. J. Am. Chem. Soc. 2003;125:10782. doi: 10.1021/ja036687f. [DOI] [PubMed] [Google Scholar]; (c) Brown SD, Peters JC. J. Am. Chem. Soc. 2004;126:4538. doi: 10.1021/ja0399122. [DOI] [PubMed] [Google Scholar]; (d) Brown SD, Peters JC. J. Am. Chem. Soc. 2005;127:1913. doi: 10.1021/ja0453073. [DOI] [PubMed] [Google Scholar]
- 4.Fe(IV) imides have been implicated as intermediates in nitrene transfer reactions: Lucas RL, Powell DR, Borovik AS. J. Am. Chem. Soc. 2005;127:11596. doi: 10.1021/ja052952g. Jensen MP, Mehn MP, Que L., Jr Angew. Chem. Int. Ed. 2003;42:4357. doi: 10.1002/anie.200351605.
- 5.For recent non-heme iron examples see: Rohde J-U, In J-H, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L., Jr Science. 2003;299:1037. doi: 10.1126/science.299.5609.1037. Klinker EJ, Kaiser J, Brennessel WW, Woodrum NL, Cramer CJ, Que L., Jr Angew. Chem. Int. Ed. 2005;44:3690. doi: 10.1002/anie.200500485. Bukowski MR, Koehntop KD, Stubna A, Bominaar EL, Halfen JA, Münck E, Nam W, Que L., Jr Science. 2005;310:1000. doi: 10.1126/science.1119092. Grapperhaus CA, Mienert B, Bill E, Weyhermuller T, Wieghardt K. Inorg. Chem. 2000;39:5306. doi: 10.1021/ic0005238.
- 6.Verma AK, Nazif TN, Achim C, Lee SC. J. Am. Chem. Soc. 2000;122:11013. [Google Scholar]
- 7.A related bis(pyrazole)(phosphine)borate ligand was reported recently: Casado MA, Hack V, Camerano JA, Ciriano MA, Tejel C, Oro LA. Inorg. Chem. 2005;44:9122. doi: 10.1021/ic051279t.
- 8.In this paper we adopt the ligand abbreviations [PhBPtBu 2(pz)] and [PhBPtBu2(pzMe2)] to refer to a parent pyrazole and a 3,5-dimethyl substituted derivative, respectively. [PhBPtBu 2(pz’)] is a general notation to represent any pyrazole derivative
- 9.(a) Sur SK. J. Magn. Reson. 1989;82:169. [Google Scholar]; (b) Evans DF. J. Chem. Soc. 1959:2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed experimental procedures for 1, 2a,b – 6a,b, all characterization data, and crystallographic details for 3a, 5a, 5b, 6a, and 6b. This material is available free of charge via the Internet at http://pubs.acs.org.