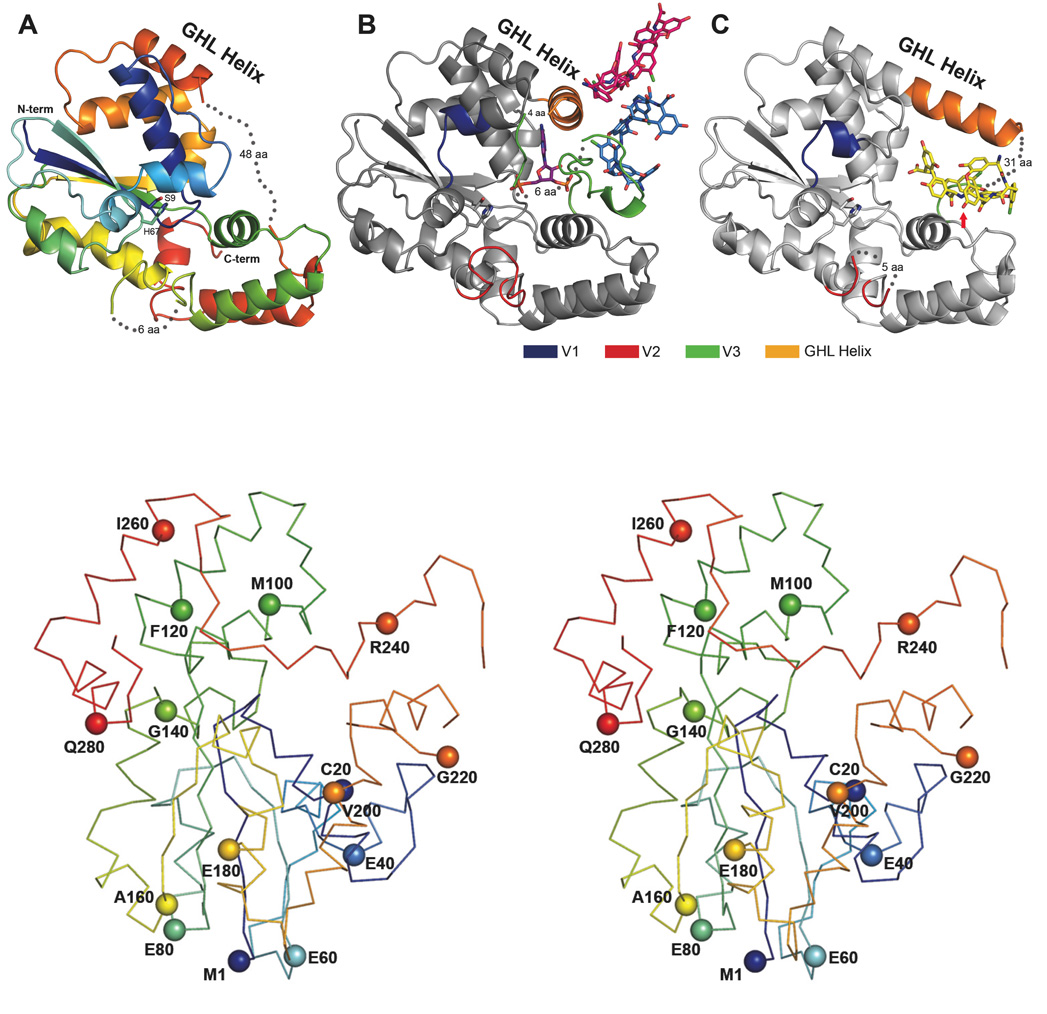
Figure 2.
The Three Teg12 structures are presented. Only a single monomer from the dimer is shown for clarity. (A) Teg12-Apo is colored using a rainbow scheme, from blue (N-term) to red (C-term). Regions of disorder are connected with grey dots, with the number of disordered residues indicated. The flexible GHL Helix is shown in orange. Side chains for the proposed active site residues His67 and Ser9 are also shown. (B) Teg12-ternary complex containing PAP and the teicoplanin aglycone. The protein has been colored grey in order to accentuate regions of the structure that differ from Teg12-Apo. Specifically, these regions are the variable loops V1–V3, colored blue, red, and green, respectively. Also shown is a molecule of PAP in the active site, a molecule of teicoplanin aglycone that interacts with the V3 loop (colored sky blue), and an additional molecule of teicoplanin aglycone involved in crystal packing interactions (colored hot pink). (C) Teg12-binary structure complexed with teicoplanin aglycone in the active site cavity. Again, the protein has been colored grey with V1–V3 colored as in the ternary structure. The active site is shown as a stereo close-up image in figure 6. Teg12 sulfates the hydroxyl of residue 3 of the teicoplanin aglycone, which has been indicated in the figure with a red arrow. Omitted from the binary structure is a second molecule of teicoplanin aglycone bound to the outside of the protein on the opposite side of the GHL helix. (D) Stereo view of a Cα trace of the Teg12-ternary structure represented as a ribbon diagram with the Cα carbon of every 20th amino acid shown as a sphere.