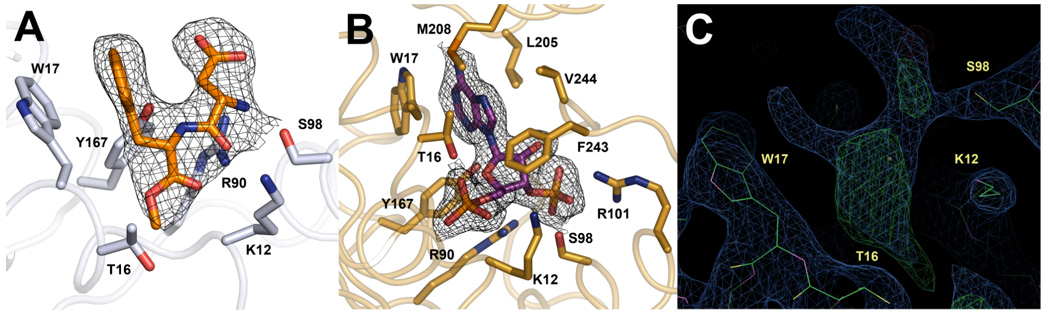
Figure 4.
Comparison of the ligands bound in the PAPS binding sites from Teg12-Apo and Teg12-ternary. Electron density from the 2fo-fc map surrounding each ligand and contoured at 1σ is shown. (A) The PAPS binding site of monomer A from Teg12-Apo contained a molecule of the dipeptide aspartame (N-(L-a-aspartyl)-L-phenylalanine,1-methyl ester). Several Teg12 residues important for coordinating PAP also make contacts with aspartame. Their side chains are shown. (B) PAP bound in the Teg12-ternary complex. Leu205 and Met208 from the GHL helix and Phe243 and Val244 from the V3 loop make significant Van der Waals contacts with PAP. (C) Electron density in the PAPS binding site of the Teg12-apo monomer A, before the addition of aspartame to the model is shown. Both the 2fo-fc (contoured at 1 sigma) (green) and the fo-fc (contoured at 3 sigma) (blue) maps are shown. Aspartame was added to the model only after the protein had been fully built, at the second to last refinement step (before the addition of waters).